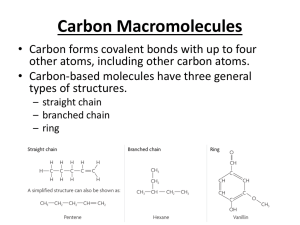
Carboxylic Acids
advertisement

Chapter 35 Carboxylic Acids and their Derivatives 35.1 35.2 35.3 35.4 35.5 35.6 35.7 1 Introduction Nomenclature of Carboxylic Acids and their Derivatives Physical Properties of Carboxylic Acids Preparation of Carboxylic Acids Reactions of Carboxylic Acids Reactions of the Derivatives of Carboxylic Acids Uses of Carboxylic Acids and their Derivatives New Way Chemistry for Hong Kong A-Level Book 3B 35.1 Introduction (SB p.29) Carboxylic acids refer to the class of organic compounds with the carboxyl group 2 New Way Chemistry for Hong Kong A-Level Book 3B 35.1 Introduction (SB p.29) General formula of carboxylic acid: RCOOH Examples: 3 New Way Chemistry for Hong Kong A-Level Book 3B 35.1 Introduction (SB p.30) Carboxylic acid derivatives Name Structure Acyl chlorides Acid anhydrides Amides Esters 4 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.30) Carboxylic Acids Carboxylic acids are named by replacing the final “-e” of the name of the corresponding alkane with “-oic acid” When other substituents are present, the carboxyl carbon is assigned position 1 Examples: 5 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.31) Acyl Chlorides Acyl chlorides are named by replacing the final “-ic acid” of the name of the parent carboxylic acid with “-yl chloride” Examples: 6 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.31) Acid Anhydrides Acid anhydrides are named by dropping the word “acid” from the name of the parent carboxylic acid and then adding the word “anhydride” Examples: 7 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.31) Amides • Amides that have no substituents on the nitrogen atom are named by replacing “-oic acid” from the parent carboxylic acid with “amide” • Substituents on the nitrogen atom of amides are named and the named substituent is preceded by N-, or N,N- Examples: 8 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.31) Esters • The names of esters are derived from the names of the alcohol (with the ending “-yl”) and the carboxylic acid (with the ending “-oate”) • The portion of the name derived from the alcohol comes first Examples: 9 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.32) Example 35-1 Give the IUPAC names of the following compounds: (a) Solution: (b) (a) 3-Methylbutanoic acid (b) N-methylethanamide (c) (c) Ethyl benzoate (d) Benzoic anhydride (d) Answer 10 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.33) Example 35-2 An ester is formed by reacting an alcohol with a carboxylic acid. Draw the structural formula of the following ester molecule and give the name of the alcohol and the carboxylic acid that form the ester. (a) Methyl ethanoate Solution: Answer (a) The structural formula of methyl ethanoate is: It is formed from the reaction of ethanoic acid and methanol. 11 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.33) Example 35-2 An ester is formed by reacting an alcohol with a carboxylic acid. Draw the structural formula of the following ester molecule and give the name of the alcohol and the carboxylic acid that form the ester. (b) Ethyl methanoate Answer Solution: (b) The structural formula of ethyl methanoate is: It is formed from the reaction of methanoic acid and ethanol. 12 New Way Chemistry for Hong Kong A-Level Book 3B 35.2 Nomenclature of Carboxylic Acids and their Derivatives (SB p.33) Check Point 35-1 Complete the following table by filling in the molecular formulae, structural formulae or IUPAC names of the (a) carboxylic acids. Molecular formula Structural formula IUPAC name (a) C3H7COOH(b) Butanoic acid (c) (c)(b) CH3CH(CH3)CH2COOH (d) 3-Methylbutanoic acid(d) (e) C6H4ClCOOH (f) 2-Chlorobenzoic acid (g) CCl3COOH (h) (e) (f) Answer (g) 13 (h) Trichloroethanoic acid New Way Chemistry for Hong Kong A-Level Book 3B 35.3 Physical Properties of Carboxylic Acids (SB p.34) • Carboxylic acids are colourless liquids at room conditions • They have characteristic pungent smell and sour taste Carboxylic acid Methanoic acid Ethanoic acid Propanoic acid Butanoic acid Pentanoic acid Hexanoic acid Benzoic acid Phenylethanoic acid 2-Methylpropanoic acid Chloroethanoic acid 14 Formula HCO2H CH3CO2H CH3CH2CO2H CH3(CH2)2CO2H CH3 (CH2)3CO2H CH3 (CH2)4CO2H C6H5CO2H C6H5CH2CO2H (CH3)2CHCO2H CH2ClCO2H Boiling Melting Density at point (°C) point (°C) 20°C (g cm–3) 101 118 141 164 186 205 249 266 154 189 New Way Chemistry for Hong Kong A-Level Book 3B 8.4 16.6 –20.8 –6.5 –34.5 –1.5 122 76 –47 63 1.220 1.049 0.992 0.964 0.939 0.927 — — 0.950 — 35.3 Physical Properties of Carboxylic Acids (SB p.34) Carboxylic acid Dichloroethanoic acid Trichloroethanoic acid Hydroxyethanoic acid 2-Hydroxypropanoic acid Cis-Butenedioic acid Trans-Butenedioic acid Ethanedioic acid Propandioic acid Butanedioic acid Benzene-1,2-dicarboxylic acid 15 Formula Density Boiling Melting at 20°C point (°C) point (°C) (g cm–3) CHCl2CO2H 194 CCl3CO2H 198 HOCH2CO2H dec. CH3CHOHCO2H dec. HO2CCH = CHCO2H dec. HO2CCH = CHCO2H dec. HO2CCO2H — HO2CCH2CO2H dec. HO2C(CH2)2CO2H 235 (dec.) C6H4(CO2H)2 dec. New Way Chemistry for Hong Kong A-Level Book 3B 10.8 57.7 79 16.8 130 – 131 268 (dec.) 190 (dec.) 136 (dec.) 182 206 (dec.) 1.566 — — 1.249 1.590 1.635 — — — — 35.3 Physical Properties of Carboxylic Acids (SB p.35) Boiling Point and Melting Point Due to formation of intermolecular hydrogen bonds high b.p. and m.p. The hydrogen bonds are more extensive than those in alcohols higher b.p. and m.p. than alcohols 16 New Way Chemistry for Hong Kong A-Level Book 3B 35.3 Physical Properties of Carboxylic Acids (SB p.36) Density • The densities of carboxylic acids decrease with increasing relative molecular masses • Only methanoic acid and ethanoic acid are denser than water at 20°C ∵ closer packing of the smaller molecules in the liquid phase 17 New Way Chemistry for Hong Kong A-Level Book 3B 35.3 Physical Properties of Carboxylic Acids (SB p.36) Solubility • Carboxylic acids of low molecular masses show appreciable solubilities in water ∵ carboxylic acids are polar and can form strong hydrogen bonds with water molecules • First four carboxylic acids are miscible with water • The length of the hydrocarbon portion solubilities in water 18 New Way Chemistry for Hong Kong A-Level Book 3B 35.3 Physical Properties of Carboxylic Acids (SB p.36) Example 35-3 (a) Propanoic acid has a boiling point of 141°C which is considerably higher than that of butan-1-ol (117°C), although they have the same molecular mass. Explain why. Answer Solution: (a) Each propanoic acid molecule forms two intermolecular hydrogen bonds with another propanoic acid molecule. However, two butan-1-ol molecules form only one hydrogen bond. As molecules of propanoic acid form more extensive intermolecular hydrogen bonds than those of butan-1-ol, the boiling point of propanoic acid is higher than that of butan-1-ol. 19 New Way Chemistry for Hong Kong A-Level Book 3B 35.3 Physical Properties of Carboxylic Acids (SB p.36) Example 35-3 (b) Rank the following compounds in decreasing order of solubility in water: CH3CH2CH2COOH, CH3CH2COOCH3, CH3COOH Answer Solution: (b) The solubility of the compounds in water decreases in the order: CH3COOH > CH3CH2CH2COOH > CH3CH2COOCH3 20 New Way Chemistry for Hong Kong A-Level Book 3B 35.3 Physical Properties of Carboxylic Acids (SB p.36) Example 35-3 (c) Propanedioic acid forms intramolecular hydrogen bonds. Draw its structural formula showing clearly the formation of intramolecular hydrogen bonds. Solution: (c) 21 New Way Chemistry for Hong Kong A-Level Book 3B Answer 35.4 Preparation of Carboxylic Acids (SB p.37) Hydrolysis of Nitriles • 2-hydroxyalkanenitriles is formed by nucleophilic addition reaction of aldehydes or ketones with hydrogen cyanide • Acid hydrolysis or alkaline hydrolysis of 2-hydroxyalkanenitriles produce carboxylic acids or carboxylate ions respectively 22 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.37) Nitriles can be formed by nucleophilic substitution reaction of haloalkanes with sodium cyanide Acid hydrolysis of the nitrile produces a carboxylic acid with one more carbon atom e.g. 23 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.38) Hydrolysis of Esters • Hydrolysis of esters give parent carboxylic acids and alcohols by boiling under reflux with the dilute HCl or dilute H2SO4 • Acyl chlorides, acid anhydrides, esters and amides can be hydrolyzed to give the corresponding carboxylic acids • Reactive derivatives react vigorously with water, e.g. acyl chlorides • Less reactive derivatives require more severe conditions to bring about the reaction 24 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.38) Oxidation of Alcohols and Aldehydes Aldehydes and 1° alcohols can be oxidized to carboxylic acids by KMnO4/H+ 25 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.38) Examples: 26 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.39) Oxidation of Alkybenzenes 1° and 2° alkyl groups (but not 3°) directly attached to a benzene ring are oxidized by hot KMnO4/OH– to carboxyl group e.g. This oxidation takes place initially at the benzylic carbon alkyl groups longer than methyl group are ultimately degraded to benzoic acid 27 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.39) Check Point 35-2 Write the equations for the acid-catalyzed and basecatalyzed hydrolyses of each of the following substances: (a) (a) Ethyl butanoate Answer 28 New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.39) Check Point 35-2 Write the equations for the acid-catalyzed and basecatalyzed hydrolyses of each of the following substances: (b) Propanamide (b) 29 Answer New Way Chemistry for Hong Kong A-Level Book 3B 35.4 Preparation of Carboxylic Acids (SB p.39) Check Point 35-2 Write the equations for the acid-catalyzed and basecatalyzed hydrolyses of each of the following substances: (c) Benzoyl chloride (c) Answer 30 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.39) Acidity of Carboxylic Acids • Carboxylic acids are weak acids • Their acidic properties are due to the presence of ionizable hydrogen atom 31 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.39) e.g. ethanoic acid The acid strength of ethanoic acid is shown by the value of its acid dissociation constant (Ka) [CH 3COO- ][H 3O ] Ka [CH 3COOH ] pKa is used for convenience as Ka is small for weak acids pKa = –log Ka , the smaller the pKa value, the stronger is the acid 32 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.40) • Unsubstituted carboxylic acids have pKa values in the range of 3 to 5 • Alcohols have pKa values in 15 to 18 carboxylic acids are much more acidic than alcohols Name 33 Formula pKa Methanoic acid HCOOH 3.75 Ethanoic acid CH3COOH 4.76 Propanoic acid CH3CH2COOH 4.87 Butanoic acid CH3CH2CH2COOH 4.82 Benzoic acid C6H5COOH 4.20 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.40) Resonance Effect The greater stability of carboxylic acids is attributed to resonance stabilization of the carboxylate ions Example: 34 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.41) • By the resonance effect, the ethanoate ion can be stabilized by spreading the negative charge over two oxygen atoms • No stabilizing resonance structures for alkoxide ions e.g. • Carboxylic acids are much acidic than alcohols ∵ formation of more stable conjugate base 35 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.41) Inductive Effect • Both compounds contain the highly polarized O – H bond due to the difference in electronegativity • The greater acidity of ethanoic acid is due to the presence of the powerful electron-withdrawing carbonyl group 36 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.41) Two resonance structures for the carbonyl group: • The carbonyl atom of ethanoic acid bears a large partial positive charge and exerts a powerful electronwithdrawing inductive effect to hydroxyl oxygen atom, making the hydroxyl hydrogen atom much more positive proton dissociate more readily • The electron-withdrawing inductive effect of the carbonyl group stabilizes the carboxylate ion carboxylic acids are stronger acid than alcohols 37 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.42) Inductive Effects of Other Groups 1. Electron-withdrawing Groups • The presence of electron-withdrawing groups increase the acidity of a carboxylic acid e.g. 38 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.42) • The chlorine atom withdraws electron from the carbonyl group and oxygen making the hydroxyl hydrogen more positive than that of ethanoic acid • The chlorine atom also stabilizes the chloroethanoate ion to greater extent 39 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.41) Effect of electron-withdrawing substituents on the acidity of carboxylic acids Acidity CH3COOH < Ethanoic Chloroethanoic acid acid pKa 4.76 2.86 Acidity CH3COOH < < pKa < < Dichloroethanoic acid 1.29 < CCl3COOH Trichloroethanoic acid 0.65 < Ethanoic Iodoethanoic Bromoethanoic ChloroethanoicFluoroethanoic acid acid acid acid acid 4.76 3.17 2.90 2.86 2.66 • The greater the number of electron-withdrawing groups, the stronger is the acid 40 • The more electronegative of halogen substituents (F > Cl > Br > I), the stronger is the acid New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.43) • The further away of the substituents from the carboxyl group, the weaker is the acid Acidity < pKa 41 4-Chlorobutanoic acid 4.52 < 3-Chlorobutanoic acid 4.06 New Way Chemistry for Hong Kong A-Level Book 3B 2-Chlorobutanoic acid 2.84 35.5 Reactions of Carboxylic Acids (SB p.43) 2. Electron-releasing Groups • The presence of electron-releasing groups reduce the acidity of a carboxylic acid e.g. 42 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.43) • Electron-releasing alkyl groups release electrons towards the electron-deficient carbonyl carbon, thus reducing its charge • Reduce the positive character of the hydroxyl hydrogen atom dissociate less readily • Intensify the negative charge on the carboxyl group destablizes the ethanoate ion 43 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.44) Check Point 35-3 (a) Match the Ka values: 2.19 10–3 M and 1.26 10–3 M, to the carboxylic acids: CH2FCOOH and CH2BrCOOH. Explain your answer briefly. (a) The greater the Ka value, the stronger is the acid.Answer CH2FCOOH is a stronger acid than CH2BrCOOH. This –F substituent exerts a stronger inductive effect than the –Br substituent, as fluorine is more electronegative than bromine. The –F substituent stabilizes the conjugate base (i.e. CH2FCOOH–) to a greater extent than the –Br substituent. Therefore, CH2FCOOH has a Ka value of 2.19 10–3 M, and CH2BrCOOH has a Ka value of 1.26 10–3 M. 44 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.44) Check Point 35-3 (b) Arrange the following acids in ascending order of acidity: CHCl2COOH, CCl3COOH, CH2ClCOOH, CH3COOH Explain the order briefly. Answer (b) CH3COOH < CH2ClCOOH < CHCl2COOH < CCl3COOH The –Cl substituent is an electron-withdrawing group. An increasing number of the –Cl substituent brings about a greater negative inductive effect. Thus, the conjugate base will be stabilized more, and the acidity of the acid increases. 45 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.44) Check Point 35-3 (c) Arrange the following aryl carboxylic acids in descending order of acidity: Explain the order briefly. 46 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.44) (c) is more acidic than ,because contains the –Cl substituent which is an electron-withdrawing group. Thus, it exerts a negative inductive effect to the conjugate base, and hence stabilizes the carboxylate ion formed. is less acidic than , because contains a methyl group which is an electron-releasing group. Thus, it exerts a positive inductive effect to the conjugate base, and hence destabilizes the carboxylate ion formed. 47 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.44) Check Point 35-3 (d) Which is a stronger acid, CH2ClCOOH or CH3CHClCOOH? Explain your answer briefly. Answer (d) CH2ClCOOH is a stronger acid as CH3CHClCOOH contains a methyl group which is an electron-releasing group. It exhibits a positive inductive effect and thus destabilizes the conjugate base of the acid. Therefore, CH3CHClCOOH is less acidic. 48 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.44) Formation of Salts Reaction with Active Metals • Carboxylic acids react with reactive metals such as Na or Mg to give the corresponding metal carboxylates and hydrogen gas 49 New Way Chemistry for Hong Kong A-Level Book 3B Example: 50 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.45) Reaction with Bases Carboxylic acids react with strong alkalis such as NaOH to form sodium carboxylates and water Examples: 51 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.45) Carboxylic acids also react weak alkalis such as Na2CO3 or NaHCO3 to form sodium carboxylates, carbon dioxide and water 52 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.45) Examples: • This reaction serves as a test to distinguish carboxylic acids and other acidic organic compounds 53 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.46) NaOH and Na2CO3 can be used to separate a mixture of alcohols, phenols and carboxylic acids 54 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.46) Check Point 35-4 (a) Describe how to separate a mixture of benzoic acid and phenol in the laboratory. Answer (a) Sodium hydrogencarbonate, water and diethyl ether are added to the mixture. Benzoic acid reacts with sodium hydrogencarbonate to form a water-soluble salt which dissolves in the aqueous layer. After separation by the use of a separating funnel, phenol can be recovered by distilling off the ether. To the aqueous layer, hydrochloric acid is added to generate the benzoic acid which can be extracted by diethyl ether. After separation from the aqueous layer, benzoic acid is obtained by distilling off the ether. 55 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.46) Check Point 35-4 (b) Outline how a mixture of butanone and ethanoic acid can be separated in the laboratory. Answer 56 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.46) (b) 57 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.47) Formation of Acyl Chlorides • Acyl chlorides are the most reactive amongst the carboxylic acid derivatives • They can be prepared by the use of SOCl2, PCl3 or PCl5 in good yields 58 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.47) • Acyl chlorides can be used to prepare aldehydes, ketones, esters, amides and acid anhydrides in organic synthesis • Acyl chlorides are extremely sensitive to moisture, therefore, it must be stored in anhydrous conditions ∵ hydrolyze rapidly to form carboxylic acids 59 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.47) Formation of Acid Anhydrides • Acid anhydrides can be prepared by reacting acyl chlorides with carboxylic acids in the presence of pyridine • This can be used to prepare mixed anhydrides (R R´) or simple anhydrides (R = R´) 60 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.47) • Acyl chlorides react with sodium salts of carboxylic acids to give acid anhydrides 61 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.48) Formation of Amides • Ammonia reacts with carboxylic acids to form ammonium salts. The dry salts are heated to dehydrate to give amide • The better way to prepare amides is reacting ammonia or amines with acyl chlorides 62 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.48) Formation of Esters • Carboxylic acids react with alcohols to form esters through condensation reactions • Esterification reactions are acid-catalyzed and reversible 63 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.48) Reduction with Lithium Tetrahydridoaluminate LiAlH4 is a powerful reducing agent which reduces carboxylic acids to primary alcohols in good yields Example: 64 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.49) Example 35-4 (a) Complete and balance the following acid-base Solution: equations: (a) (i)(i) (ii) (ii) Answer 65 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.49) Example 35-4 (b) Draw the structural formulae for the products formed from the following compounds when they are reduced with lithium tetrahydridoaluminate. (i) (ii) Solution: (b) (i) Answer (ii) HOCH2CH2CH2CH2OH 66 New Way Chemistry for Hong Kong A-Level Book 3B 35.5 Reactions of Carboxylic Acids (SB p.49) Example 35-4 Solution:the following equations: (c) Complete (i) (c) (i) (ii) (ii) (iii) (iii) Answer 67 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.50) Reactions of Acyl Chlorides • Both chlorine and oxygen atoms are electronwithdrawing groups, thus making the acyl carbon much electron-deficient acyl carbon is a good nucleophilic site • Chloride ion is a good leaving group which is substituted by other atoms or groups easily acyl chlorides are very reactive 68 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.50) • Benzoyl chloride is much less reactive than aliphatic acyl chlorides ∵ reduction of electron-deficiency on the acyl carbon due to delocalization of electrons (resonance effect) 69 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.51) Reaction with Water Acyl chlorides are hydrolyzed by water to form the parent carboxylic acids and hydrogen chloride Examples: 70 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.51) Reaction with Alcohols Acyl chlorides react with alcohols to give esters and HCl Examples: 71 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.51) Acyl chlorides react with phenols to give esters in the presence of base as catalyst ∵ an alkaline medium converts phenol to a powerful nucleophile, phenoxide ion Example: 72 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.52) Reaction with Ammonia and Amines Acyl chlorides react with ammonia to form amides rapidly Example: 73 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.52) Acyl chlorides react rapidly with 1° and 2° amines to form N-, N,N- substituted amides respectively • The reaction takes place at room temperature and produces amides in high yield • This method is widely used in laboratory for amides synthesis 74 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.52) Check Point 35-5 Explain why ethanoyl chloride must be protected from atmospheric moisture during storage. Answer This is because ethanoyl chloride reacts readily with water (from atmospheric moisture) to form ethanoic acid. 75 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.53) Reactions of Acid Anhydrides Reaction with Water Acid anhydrides undergo hydrolysis to form carboxylic acids Example: 76 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.53) Reaction with Alcohols Acid anhydrides react with alcohols to form esters in the presence of acid catalyst Example: 77 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.54) Reaction with Ammonia and Amines Acid anhydrides react with ammonia and with 1° and 2° amines in the ways similar to those of acyl chlorides 78 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.54) Examples: 79 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.53) Reactions of Amides Amides are the least reactive among the carboxylic acid derivatives towards nucleophilic substitution reactions ∵ NH2–, NHR– or NR2– are strong bases and thus poor leaving groups 80 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.55) Reaction with Water Amide undergo acid and alkaline hydrolyses to form carboxylic acids and carboxylates respectively Acid hydrolysis: Alkaline hydrolysis: 81 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.55) Dehydration Amides are dehydrated by heating with P4O10 to form nitriles • Useful synthetic method for preparing nitriles that are not available by nucleophilic substitution reactions between haloalkanes and cyanide ions 82 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.55) Hofmann Degradation • Amides react with a solution of Br2 in NaOH or a solution of Cl2 in NaOH to give amines through a reaction called Hofmann degradation • The resulted amines have one carbon less than original amides Example: 83 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.56) Reduction with Lithium Tetrahydridoaluminate Amides are reduced by LiAlH4 in dry ether to give 1° amines 84 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.56) Reactions of Esters Acid-catalyzed Hydrolysis Acid-catalyzed hydrolysis of esters is the reverse reaction of esterification Example: 85 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.56) Alkali-catalyzed Hydrolysis • When esters are refluxed with an alkali such as NaOH, the corresponding alcohol and sodium salt of the carboxylic acid are produced • Alkali-catalyzed hydrolysis are irreversible Example: 86 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.57) • Soap is made by the alkaline hydrolysis of fats or oils (i.e. triesters) to produce sodium carboxylates (i.e. soap) • The reaction is called saponification 87 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.57) Reduction with Lithium Tetrahydridoaluminate Esters are reduced to alcohols by LiAlH4 Example: 88 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.57) Example 35-5 Draw the structural formulae of the missing compounds A to Solution: M: (a) (a) (b) (b) (c) (c) Answer 89 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.58) Example 35-5 Draw theSolution: structural formulae of the missing compounds A to M: (d) (d) (e) (e) (f) (f) Answer 90 New Way Chemistry for Hong Kong A-Level Book 3B 35.6 Reactions of the Derivatives of Carboxylic Acids (SB p.58) Example 35-5 Draw the structural formulae of the missing compounds A to M: Solution: (g) (h) (i) (g) (h) (i) Answer 91 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.59) As Food Preservatives • Benzoic acid and its sodium salt used as food preservatives • Prevent the microbial growth and spoilage • Used in oyster sauce, fish sauce, ketchup, non-alcoholic beverages, fruit juices, margarine, salad dressings, jams and pickled products 92 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.59) As Raw materials for Making Polymers • Nylon-6,6, condensation polymer ,is a • It is a polyamide made by adding NaOH containing hexanedioyl dichloride and hexane-1,6-diamine to CH3CCl3 93 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.60) Advantages of Nylon-6,6 • Drip dry easily • Not attack by insects easily • Resist creasing (no ironing required after washing) • Used for making ropes, thread, cords, and various kinds of clothing from stockings to jackets 94 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.60) Terylene, , is a polyester made from acid-catalyzed condensation polymerization of benzene-1,4-dicarboxylic acid and ethane-1,2-diol Terylene is used to make washand-wear garments 95 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.60) As Solvents Liquid esters are widely used as solvents for all-purpose adhesives, thinners for paints and nail varnish removers 96 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.60) As Flavourings • Volatile esters often have characteristic sweet and fruity smells • They are used as artificial flavourings in food-processing industry An artificial flavouring made of esters 97 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.60) Some esters and their characteristic flavours Structure of ester Characteristic flavour Banana Apple Apricot Pineapple 98 New Way Chemistry for Hong Kong A-Level Book 3B 35.7 Uses of Carboxylic Acids and their Derivatives (SB p.60) Some esters and their characteristic flavours (cont’d) Structure of ester Characteristic flavour Rum Grape Wintergreen 99 New Way Chemistry for Hong Kong A-Level Book 3B The END 100 New Way Chemistry for Hong Kong A-Level Book 3B