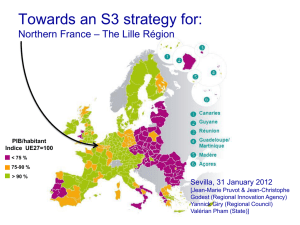
A.Lisowska
advertisement
Electrochemical systems for energy storage devices A. Lisowska-Oleksiak, A.P. Nowak, M. Wilamowska, K. Szybowska Gdansk University of Technology, Chemical Faculty Narutowicza 11/12, 80-233 Gdańsk International EcoEnergy Clusters Meeting | 12.05.2010 | Energy sources can be divided into three broad categories Chemical (oxidizing some reduced substance) or photophysical energy (absorbing sunlight to generate either heat or electricity) Nuclear reactions (splitting heavy nuclei or by fusing light nuclei) Thermomechanical (wind, water, or geological sources of steam or hot water) International EcoEnergy Clusters Meeting | 12.05.2010 | Steps in electric energy consume: 1) Generation 2) Transmission 3) Convertion 4) Storage (mechanical, chemical, and thermal) 5) Consumption International EcoEnergy Clusters Meeting | 12.05.2010 | The storage techniques can be divided into four categories 1) Low-power application in isolated areas, essentially to feed transducers and emergency terminals, 2) Medium-power application in isolated areas (individual electrical systems, town supply), 3) Network connection application with peak leveling, 4) Power-quality control applications. International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems (for high and medium power application) Pumped hydro storage (PHS) – uses for high power applications with 60-85% of conversion efficiency Pump-storage power station in Żarnowiec International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems (for high and medium power application) Compressed air energy storage (CAES) – high power applications, energy density ~ 12 kWh/m3 with efficiency 70% International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems (for high and medium power application for peak leveling) Energy storage using flow batteries (FBES) Regenesys Technologies (England) ~ 120MWh with 75% effficiency International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems (for low and medium power application) Fuel cells – Hydrogen energy storage (FC– HES) Main components: 1) Electrolyzer (to produce hydrogen), 2) Fuel cell (to consume hydrogen), 3) tank (to store hydrogen if needed) Alkaline Fuel Cell (AFC), Polymer Exchange Membrane Fuel Cell (PEMFC), Direct Methanol Fuel Cell (DMFC), Phosphoric Acid Fuel Cell (PAFC), Molten Carbonate Fuel Cell (MCFC), Solid Oxide Fuel Cell (SOFC) FC-HES is a low-efficiency solution: Electrolyzer (70%) The fuel cell (50%) Total efficiency ~ 35% International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems (for low and medium power application) Chemical storage - transform chemical energy into electrical energy using Faradaic process Ox + ne- = Red Batteries - Primary (source of the energy) - Secondary (storage and source of the energy) International EcoEnergy Clusters Meeting | 12.05.2010 | Batteries (lead–acid, nickel–cadmium, nickel–metal hydride, nickel–iron, zinc–air, iron–air, lithium–ion, lithium–polymer, etc.) (+) high energy densities up to 200 Wh/kg (lithium) (-) low cycleability (up to 4000 cycles) International EcoEnergy Clusters Meeting | 12.05.2010 | sodium–sulphur, Electricity storage systems Lithium and Lithium-ion batteries (for 3 C technologies) Item Panasonic (cylindrical) Panasonic (prismatic) Nominal voltage 3.6 – 3.7 V 3.6 – 3.7 V Nominal capacity 720 – 3100 mAh 920 – 1950 mAh Mass 18 – 95 g 16 – 39 g Item Sony (Li-Ion) Sony (Li-polymer) Nominal voltage 2.5 – 4.2 V 3.0 – 4.2 V Nominal capacity 1600 - 2550 mAh 830 – 1050 mAh Mass 44 – 90 g 14.3 – 22.5 g International EcoEnergy Clusters Meeting | 12.05.2010 | Item A123Systems (cylindrical) A123Systems (prismatic) Nominal voltage 3.3 V 3.3 V Nominal capacity 1100 – 2300 mAh 20 Ah Mass 39 – 70 g - Electricity storage systems Lithium and Lithium-ion batteries in the future Nowadays the challenge is to obtain material for high power and high energy application able to be used in electric vehicles http://www.treehugger.com/files/2008/02/lithium-ion_battery_factory.php International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems Lithium-ion batteries (materials) Specific capacity [mAh/g] Potential [V] LiCoO2 155 3.5 – 4.3 LiMn2O4 140 3.7 – 4.3 Li(Co,Ni)yMn2yO4 160 4.5 – 5.0 LiMnPO4 150 3.6 – 4.4 LiFePO4 170 3.0 – 3.3 LiNixCoyAlzO2 180 3.6 – 4.2 graphite 350 0.1 – 0.22 hard carbons > 350 0.6 lithium 3800 0 Li4Ti5O12 155 1.5 Li4.4Si 4200 0.3 LiSiCN 550 0.1 – 0.4 cathode anode International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems Chemical storage (Photovoltaic cells) - transform solar energy into electrical energy Problem: To store excess of the energy in one device!!! International EcoEnergy Clusters Meeting | 12.05.2010 | Electricity storage systems Bifunctional TiO2 for energy storage Materials: WO3, MoO3, phosphotungstic acid (PWA), Mechanism of energy storage of TiO2/WO3 composite system International EcoEnergy Clusters Meeting | 12.05.2010 | Schematic Diagram of the Photoelectrolysis Cell for Hydrogen Generation International EcoEnergy Clusters Meeting | 12.05.2010 | ‘I believe that water will one day be used as a fuel because the hydrogen and oxygen which constitute it, used separately or together, will furnish an inexhaustible source of heat and light. I therefore believe that, when coal deposits are oxidised, we will heat ourselves by means of water. Water is the coal of the future’ ‘L’Ile Mysterieuse’, Jules Verne 1875, International EcoEnergy Clusters Meeting | 12.05.2010 | Vis Mehcf TiO2 Current collector Combine photoanode system UV DEUV=0.15 V DEVis=1.15 V International EcoEnergy Clusters Meeting | 12.05.2010 | hv DEUV-Vis=1.30 V Electricity storage systems Electrochemical capacitors – store energy in the form of an electric field Electrochemical capacitors electrochemical double layer capacitors (EDLC) International EcoEnergy Clusters Meeting | 12.05.2010 | pseudo–capacitors electrochemical double layer capacitors (EDLC) - store energy using ion adsorption (no faradaic (redox) reaction) - high specific surface area (SSA) electrodes (carbon) 100 – 120 F/g (nonaqueous electrolyte) 150 – 300 F/g (aqueous electrolyte) International EcoEnergy Clusters Meeting | 12.05.2010 | pseudo–capacitors (store energy using fast surface redox reactions ) - redox reaction occurs at the surface of the active material (metal oxides (RuO2, Fe3O4, MnO2), conducting polymers (polyaniline, polypyrrole, polythiophene etc.) Materials Metal oxides: Capacity 1300 F/g (RuO2) Nominal voltage 1.2 V International EcoEnergy Clusters Meeting | 12.05.2010 | Conducting polymers: Capacity 30 – 40 mAh/g Nominal voltage 1.0 V Electrochemical capacitor Battery Charge time 70% charged in seconds hours Discharge time short long Charge/discharge cycles 10000-1000000 500-1000 Pollutants metals International EcoEnergy Clusters Meeting | 12.05.2010 | none Supercapacitor International EcoEnergy Clusters Meeting | 12.05.2010 | Battery Electricity storage systems pseudo–capacitors (hybrid systems consisted of organic and inorganic conducting materials, e.g. poly(3,4-ethylenedioxythiophene) modified with transition metal hexacyanoferrate* * ~ 90 F/cm3 Micro-nanoporous pEDOT** 100 F/cm3 * M. Wilamowska, A. Lisowska-Oleksiak, J. Power Sources, 194 (2009) 112-117 ** Snook et al. Electrochem Commun., 9 (2007) 83-88 International EcoEnergy Clusters Meeting | 12.05.2010 | Supercapacitors – alternative way for public transport Prototype Shanghai super-capacitor electric bus at a recharging station Costs ~ 8000 € (after 12 years one may save 160 000 €) Speed (max) 45 km/h Capacity 6 Wh/kg Distance (max) 5-9 km Charging time 5-10 min http://www.citytransport.info/Electbus.htm International EcoEnergy Clusters Meeting | 12.05.2010 | Supercapacitors for wind power station International EcoEnergy Clusters Meeting | 12.05.2010 | Supercapacitors for solar power station Production Application Supercapacitors International EcoEnergy Clusters Meeting | 12.05.2010 | Summary 3.6 s Compresed air energy storage 1 hour Pumped hydro storage 41 days Batteries Flow batteries Supercapacitors International EcoEnergy Clusters Meeting | 12.05.2010 | International EcoEnergy Clusters Meeting | 12.05.2010 | Our laboratory members Prof. A. Lisowska-Oleksiak and the team International EcoEnergy Clusters Meeting | 12.05.2010 |