Practice Problem
advertisement

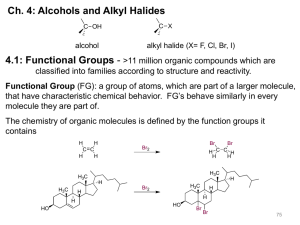
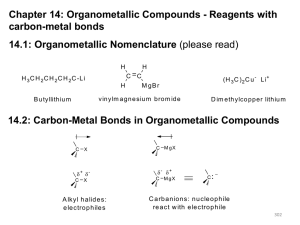
Chapter 10 Introduction • Alkyl halides – are compounds containing halogen bonded to a saturated, sp3-hybridized carbon atom – are haloalkanes Properties and some uses It is a pain reliever It is more potent than morphine A. Naming Alkyl Halides • Alkyl halides are named according to the system of nomenclature devised by the International Union of Pure and Applied Chemistry (IUPAC): Steps to naming alkyl halides1 1. Find the parent hydrocarbon • Include the double or triple bond if present 2. Number the atoms in the main chain a. The correct sequence is when the substituents have the lowest possible number b. Use multiplicity prefixes di-, tri-, tetra-, … c. Put the substituents in alphabetical order (Do not consider multiplier prefixes) Steps to naming alkyl halides2 3. If the parent chain can be properly numbered from either end by step 2, begin at the end nearer the substituent that has alphabetical precedence 2,4,5 3,4,6 2,4,5 3,4,6 2,3,4 1,3,4 3,4,5 2,3,5 Practice Problem: Give IUPAC names for the following alkyl halides: Practice Problem: Draw structures corresponding to the following IUPAC names: a) 2-Chloro-3,3-dimethylhexane b) 3,3-Dichloro-2-methylhexane c) 3-Bromo-3-ethylpentane d) 1,1-Dibromo-4-isopropylcyclohexane e) 4-sec-Butyl-2-chlorononane f) 1,1-Dibromo-4-tert-butylcyclohexane B. Structure of Alkyl Halides • As you go down the periodic table, – C-X bond is longer – C-X bond is weaker • Due to DEN, C-X bond is polarized with: – slight positive on carbon and – slight negative on halogen • The C-X carbon atom can behave as an electrophile C. Preparing Alkyl Halides • Alkyl halide - is synthesized from addition of HCl, HBr, HI to alkenes to give Markovnikov product • Alkyl dihalide - is synthesized from anti addition of Br2 or Cl2 Reaction of Alkanes with Halogens • Alkane + Cl2 or Br2, heat or light, replaces C-H with C-X but gives mixtures – hard to control – via free radical mechanism • It is usually not a good idea to plan a synthesis that uses this method Mechanism of the radical chlorination of methane D. Radical Halogenation of Alkanes • Radical halogenation of alkanes is not a good method of alkyl halide synthesis because mixtures of products usually result. • If there is more than one type of hydrogen in an alkane, reactions favor replacing the hydrogen at the most highly substituted carbons 30% / 6 = 5% 70% / 4 = 17.5% Primary H’s Secondary H’s 35% / 1 = 35% Tertiary H’s 65% / 9 = 7.2% Primary H’s Relative Reactivity • Based on quantitative analysis of reaction products, relative reactivity is estimated • Order parallels stability of radicals • Increasing alkyl substitution stabilizes the transition state and radical intermediate The more stable radical forms faster • Reaction distinction is more selective with bromine than chlorine • Reaction distinction is more selective with bromine than chlorine • Reaction with Br. is much less exergonic • T.Sbromination resembles the alkyl radical more closely than does T.Schlorination Practice Problem: Draw and name all monochloro products you would expect to obtain from radical chlorination of 2-methylpentane. Which, if any, are chiral? Practice Problem: Taking the relative reactivities of 1o, 2o, and 3o hydrogen atoms into account, what product(s) would you expect to obtain from monochlorination of 2-methylbutane? What would the approximate percentage of each product be? (Don’t forget to take into account the number of each type of hydrogen) E. Allylic Bromination of Alkenes • N-bromosuccinimide (NBS) selectively brominates allylic positions – It requires light for activation – It is a source of dilute bromine atoms NBS allylic bromination Br. radical abstracts an allylic hydrogen Allylic radical forms as an intermediate and reacts with Br2 • Bromination with NBS occurs exclusively at an allylic position because: – an allylic radical is more stable than a typical alkyl radical by about 40 kJ/mol Stability Order • Allylic radical is more stable than tertiary alkyl radical F. Stability of the Allyl Radical • Allyl radical is resonance-stabilized – Allyl radical has two resonance forms – Alkyl radical has only one structure The greater the number of resonance forms, the greater the stability • Allyl radical is delocalized – The unpaired electron is spread out over an extended p orbital network – The unpaired electron is equally shared between the two terminal carbons • Allylic bromination of an unsymmetrical alkene often leads to a mixture of products less hindered • Allylic bromination can be used to convert alkenes into dienes by dehydrohalogenation with base. Practice Problem: Draw three resonance forms for the cyclohexadienyl radical Practice Problem: The major product of the reaction of methylenecyclohexane with N-bromo succinimide is 1-(bromomethyl)cyclohexene. Explain. Practice Problem: What products would you expect from the reaction of the following alkenes with NBS? If more than one product is formed, show the structures of all. G. Preparing Alkyl halides from Alcohols • Alcohols react with HX to form alkyl halides, but the reaction works well for tertiary alcohols, R3COH • Reaction of tertiary C-OH with HX is fast and effective – Add HCl or HBr gas into ether solution of tertiary alcohol – Primary and secondary alcohols react very slowly and often rearrange, so alternative methods are used Preparation of Alkyl Halides from Primary and Secondary Alcohols • Primary and secondary alkyl halides are normally prepared from alcohols using either – thionyl chloride (SOCl2) SOCl2 : ROH RCl – phosphorus tribromide (PBr3) PBr3 : ROH RBr – These reactions use mild conditions – less acidic and less likely to cause rearrangements Practice Problem: How would you prepare the following alkyl halides from the corresponding alcohols? H. Reaction of Organohalides: Grignard Reagents • Alkyl halides react with magnesium in ether solution to form organomagnesium halides, Grignard reagents (RMgX) • Reaction of RX with Mg in ether or THF • Product is RMgX – an organometallic compound (alkyl-metal bond) – R is alkyl 1°, 2°, 3°, aryl, alkenyl – X = Cl, Br, I • The carbon-magnesium bond is polarized The carbon atom is thus both nucleophilic and basic Reactions of Grignard Reagents • Many useful reactions – RMgX behaves as R– R- (carbon anions) are very strong bases – RMgX react with such weak acids as H2O, ROH, RCO2H and RNH2 to abstract H+ – RMgX + H3O+ R-H Practice Problem: Just how strong a base would you expect a Grignard reagent be? Look at Table 8.1, and then predict whether the following reactions will occur as written. (The pKa of NH3 is 35) a) CH3MgBr + H-CΞC-H b) CH3MgBr + NH3 CH4 + CH4 + H-CΞC-MgBr H2N-MgBr Practice Problem: How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a deuterated compound? I. Organometallic Coupling Reactions 1. Alkyllithium (RLi) forms from RBr and Li metal 2. RLi reacts with copper iodide to give lithium dialkylcopper (Gilman reagents) 3. Lithium dialkylcopper reagents react with alkyl halides to give alkanes 1. Alkyllithium (RLi) forms from RBr and Li metal Alkyllithiums are both nucleophiles and bases 2. RLi reacts with copper iodide to give lithium dialkylcopper (Gilman reagents) 3. Lithium dialkylcopper reagents react with alkyl halides to give alkanes Gilman reagents undergo organometallic coupling reactions with chlorides, bromides and iodides (but not fluorides) Utility of Organometallic Coupling in Synthesis • Coupling of two organometallic molecules produces larger molecules of defined structure • Aryl and vinyl organometallics are also effective • Coupling of lithium dialkylcopper molecules proceeds through trialkylcopper intermediate Practice Problem: How would you carry out the following transformations using an organocopper coupling reaction? More than one step is required in each case. J. Oxidation and Reduction in Organic Chemistry • Oxidation is a reaction that results in loss of electron density at carbon either by • bond formation between carbon and a more electronegative atom (oxygen, nitrogen, or halogen) • bond breaking between carbon and a less electronegative atom (usually hydrogen) – Not necessarily defined as loss of electrons by an atom as in inorganic chemistry Oxidation: break C-H (or C-C) and : form C-O, C-N, C-X • Organic Reduction is the opposite of oxidation. It results in increase of electron density at carbon either by • bond breaking between carbon and a more electronegative atom (oxygen, nitrogen, or halogen) • bond formation between carbon and a less electronegative atom (usually hydrogen) Reduction: form C-H (or C-C) and break C-O, C-N, C-X Halogenation Conversion of alkyl halide to alkane • Functional groups are associated with specific levels Maximum number of C-H Maximum number of C-X Practice Problem: Rank each of the following series of compounds in order of increasing oxidation level: Practice Problem: Tell whether each of the following reactions is an oxidation, a reduction, or neither. Explain your answers. Chapter 10