LOCAL ANESTHETICS - Professor Dr Ghaleb
advertisement

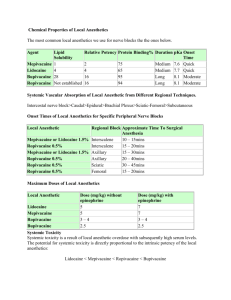
LOCAL ANESTHETICS A.Ghaleb,MD LOCAL ANESTHETICS • The electrical potential inside the cell is negative and • • close to the potential that would be determined by potassium alone. This is the resting potential (-70 mV). During the transmission of an action potential, sodium moves into the cell through open sodium channels, depolarizing the cell. Local anesthetics are compounds with the ability to interrupt the transmission of the action potential in excitable membranes. They bind to specific receptors on the Na+ channels and their action at clinically recommended doses is reversible. Historical perspective • The natives of Peru chewed coca leaves and • • knew about their cerebral-stimulating effects. The leaves of erythroxylon coca were taken to Europe where Niemann isolated cocaine in Germany in 1860. Koller in 1884 is credited with the introduction of cocaine as a topical ophthalmic local anesthetic in Austria. Cardiovascular side effects as well as potential for dependency and abuse were soon recognized, which led to the search for a better local anesthetic. Historical perspective • 1850’s invention of the syringe and hypodermic hollow needle • 1884 Halsted, blocks the brachial plexus with a solution of cocaine • • • • • under direct vision (surgical exposure). 1897 Braun in Germany relates cocaine toxicity with systemic absorption and advocates the use of epinephrine. 1898 Bier performs the first planned spinal anesthesia. 1911 Hirschel performs the first percutaneous axillary block 1911 Kulenkampff performs the first percutaneous supraclavicular block Date of introduction in clinical practice of some local anesthetics: Historical perspective • 1905 procaine; 1932 tetracaine; 1947 lidocaine; 1955 chloroprocaine (last ester type local anesthetic introduced that is still in clinical use); 1957 mepivacaine; 1963 bupivacaine; 1997 ropivacaine; 1999 levobupivacaine. Chemical structure • weak bases with a pka above 7.4 and poorly soluble in • • • water. Commercially available as acidic solutions (pH 4-7) of hydrochloride salts, which are hydrosoluble. A typical local anesthetic is composed of two portions linked together by a chemical chain. One portion consists of a benzene ring (lipid soluble “hydrophobic”) and the other is an amine group that is ionizable and watersoluble (hydrophilic). The chemical chain can be either ester type (-CO-) or amide type (-HNC-) defining two different groups of local anesthetics, esters and amides. • The injected local anesthetic volume spreads initially by mass • • • • movement. This first step determines how much local anesthetic effectively reaches the nerve. Moves across “points of least resistance”, which do not necessarily lead into the desired nerve(s), stressing the need to bring the needle in proximity to the target nerve(s). The local anesthetic solution diffuses through tissues; each layer of them acting as a physical barrier and in the process part of the solution gets absorbed into the circulation. Finally a small percentage of the anesthetic reaches the target nerve membrane at which point the different physicochemical properties of the individual anesthetic will dictate the speed, duration and nature of the interaction with the receptors. Structure-activity relationship • • • • Lipid solubility Determines both the potency and the duration of action of the local anesthetics by binding the drug close to the site of action and thereby decreasing the rate of metabolism by plasma esterase and liver enzymes. In addition the local anesthetic receptor site on Na+ channels is thought to be hydrophobic, so its affinity for hydrophobic drugs is greater. Hydrophobicity also increases toxicity, so the therapeutic index actually is decreased for more hydrophobic drugs. Structure-activity relationship • • • • • • Protein binding Related to duration of action. In the body, local anesthetics are bound in large part to plasma and tissue proteins. The bound portion is not pharmacologically active. The most important binding proteins in plasma are albumin and alpha-1-acid glycoprotein (AAG) The fraction of drug bound to protein in plasma correlates with the duration of action of local anesthetics: bupivacaine > ropivacaine > mepivacaine > lidocaine > procaine and 2-chloroprocaine. This suggests that the bond between the local anesthetic molecule and the sodium channel receptor protein may be similar to that of local anesthetic binding to plasma protein (similar amino acid sequences). Drugs as lidocaine, tetracaine and bupivacaine have been incorporated into liposomes to prolong the duration of action and decrease toxicity. Liposomes are vesicles with two layers of phospholipids, which slow down the release of the drug effectively prolonging the duration of action Structure-activity relationship • Protein binding • This suggests that the bond between the local anesthetic • molecule and the sodium channel receptor protein may be similar to that of local anesthetic binding to plasma protein (similar amino acid sequences). Drugs as lidocaine, tetracaine and bupivacaine have been incorporated into liposomes to prolong the duration of action and decrease toxicity. Liposomes are vesicles with two layers of phospholipids, which slow down the release of the drug effectively prolonging the duration of action Structure-activity relationship • • • • • The pka of the local anesthetic determines the ratio of the ionized (cationic) and the uncharged (base) form of the drug. The pka for local anesthetics ranges from 7.6 to 9.2. By definition the pka is the pH at which 50% of the drug is ionized and 50% is present as a base. The pka generally correlates with the speed of onset of most local anesthetics. The closer the pka to the physiologic pH the faster the onset (e.g., lidocaine with a pka of 7.7 is 25% nonionized at ph 7.4 and has a more rapid onset of action than bupivacaine with a pka of 8.1 which is only 15% non-ionized). One important exception is 2-chloroprocaine with a pka of 9.0 and very short onset of action. This fast onset could be related to its low toxicity, which allows for high concentrations to be used clinically. It is also claimed to have also better “tissue penetrability”. Mechanism of action and sodium channels • The non-charged hydrophobic fraction (B) crosses the • • • lipidic nerve membrane and initiates the events that lead to blocking of sodium channels. Once inside a new equilibrium, dictated by the compound pka and the intracellular pH, is reached between the non-charged and charged (BH+) fractions. Because of the relative more acidic intracellular environment, the relative proportion of charged fraction increases. This fraction interacts with the Na+ channel. Local anesthetics do not ordinarily affect the membrane resting potential. Mechanism of action and sodium channels • The Na+ channel is a protein structure that • • communicates the extracellular of the nerve with its axoplasm and consists of four repeating alpha subunits, a beta-1 and beta-2 subunits. The alpha subunits are involved in ion movement and local anesthetic activity. It is generally accepted that local anesthetics main action involves interaction with specific binding sites within the Na+ channel. The voltage–dependence of channel opening is hypothesized to reflect conformational changes in response to changes in transmembrane potential. The voltage sensors or gates are located in the S4 helix; the S4 helices are both hydrophobic and positively charged. Mechanism of action and sodium channels • The Na+ channels seem to exist in three different states, closed, • • • • open and inactive. With depolarization the protein molecules of the channel undergo conformational changes from the closed (resting) state to the ionpermeable state or open state. The channel goes then through a transitional inactive state where the proteins leave the channel still closed and ion-impermeable. With repolarization the proteins revert to their resting configuration. Local anesthetics may also block in some degree calcium and potassium channels as well as N-methyl-D-aspartate (NMDA) receptors. Other drugs like tricyclic antidepressants (amitriptyline), meperidine, volatile anesthetics and ketamine also have sodium channel-blocking properties Frequency and voltage dependence of local anesthetic action • A resting nerve is much less sensitive to local • • anesthetic than one that is being stimulated. The degree of block also depends on the nerve resting membrane potential, a more positive membrane potential causes a greater degree of block. These frequency and voltage dependent effects occur because the local anesthetic in its charged form gain access to its biding site within the channel only when the Na+ channel is in an open state Pregnancy and local anesthetics • Increased sensitivity (more rapid onset, more profound • • block) may be present during pregnancy. Also alterations in protein binding of bupivacaine may result in increased concentrations of active unbound drug in the pregnant patient. During pregnancy, placental transfer is more active for lipid soluble local anesthetics, whereas higher protein binding becomes an obstacle to such transfer. In any case, agents with a pka closer to physiologic pH have a higher placental transfer. For example the umbilical vein/maternal vein ratio for mepivacaine is 0.8 (pka 7.6) while for bupivacaine is 0.3 (pka 8.1). Pregnancy and local anesthetics • In the presence of fetal acidosis, local anesthetics cross the placenta and become ionized in higher proportion than at normal pH. As ionized substances they cannot cross back to the maternal circulation (“ion trapping”). 2chloroprocaine with its very short maternal and fetal half-lives is theoretically an ideal local anesthetic in the presence of fetal acidosis. Fiber size and pattern of blockade • As a general rule small nerve fibers are more susceptible • • • • to local anesthetics However other factors like myelinazation and relative position of the fibers (mantle and core) within a nerve also play a role. The smallest nerve fibers are nonmyelinated and are blocked more readily than larger myelinated fibers. However myelinated fibers are blocked before nonmyelinated fibers of the same diameter. In general autonomic fibers, small nonmyelinated C fibers (mediating pain), and small myelinated A delta fibers (mediating pain and temperature) are blocked before A gamma, A beta and A alpha fibers (carrying postural, touch, pressure and motor information). Fiber size and pattern of blockade • In large nerve trunks motor fibers are usually located in • the outer portion of the bundle and are more accessible to local anesthetic. Thus motor fibers may be blocked before sensory fibers in large mixed nerves. In addition the frequency-dependence of local anesthetic action favors block of small sensory fibers. They generate long action potential (5 ms) at high frequency, whereas motor fibers generate short action potentials (0.5 ms) at lower frequency. These characteristics of sensory fibers in general, and of pain fibers in particular, favor frequency-dependent block. Modulating local anesthetic action pH adjustment • Local anesthetics pass through the nerve membrane in a non• • • • ionized hydrophobic (lipid soluble) base form. In the axoplasm they equilibrate into an ionic form that is active within the sodium channel. The rate-limiting step in this cascade is penetration of the local anesthetic through the nerve membrane. All available local anesthetics contain very little drug in the nonionized state. This fraction depends on the pka of the drug and the ph of the solution. Changes in ph can produce a shortening of the onset time, being the limiting factor for ph adjustment the solubility of the base form of the drug (precipitation). DiFazio et al (Anesth Analg 1986:65; 760-64) demonstrated more than 50% decrease in onset of epidural anesthesia when the pH of commercially available lidocaine with epinephrine was raised from 4.5 to 7.2 by the addition of bicarbonate. Modulating local anesthetic action pH adjustment • Hilgier (Reg Anesth 1985:10; 59-61) reported a marked improvement in the onset time for brachial plexus anesthesia when bupivacaine with epinephrine (pH 3.9) was alkalinized to pH 6.4 before injection. • However, when only small changes in pH can be achieved because of the limited solubility of the base, only small decreases in onset time will occur, as when plain bupivacaine is alkalinized. For each local anesthetic there is a ph at which the amount of base in solution is maximal (a saturated solution). • Chloroprocaine plus 1 mL of sodium bicarbonate for 30 mL of solution raises the pH to 6.8. Adding 1 mL of sodium bicarbonate per 10 mL of lidocaine or mepivacaine raises the pH of the solution to 7.2 and adding 0.1 mL of bicarbonate per 10 mL of bupivacaine raises the pH of the solution to 6.4 Modulating local anesthetic action pH adjustment • Carbonation • Another approach to shortening onset time has been the use of carbonated local anesthetic solutions. The solution contains large amounts of carbon dioxide, which readily diffuses into the axoplasm of the nerve lowering the ph and favoring the formation of the cationic active form of the local anesthetic. Carbonated solutions are not available in the United States LOCAL ANESTHETICS ADDITIVES • Vasoconstrictors to prolong the anesthetic effect and • • • • to decrease absorption. Epinephrine is also used to detect intravascular injection (test dose). Vasoconstrictors may also improve the quality and density of the block especially with spinal and epidural anesthesia. This has been demonstrated with tetracaine, lidocaine and bupivacaine. The mechanism is unclear. Epinephrine may simply increase the amount of local anesthetic available by reducing absorption. It could have also some anesthetic effect by means of its alpha 2-agonist actions. Subarachnoid epinephrine potentially delays the time for urination, which may delay discharge. • Epinephrine used other than intrathecally is absorbed systemically and may produce adverse cardiovascular effects. • In small doses the beta-adrenergic effects predominate with increased cardiac output and heart rate. Dose larger than 0.25 mg (250 ug) may be associated with arrhythmias or other undesirable cardiac effects. • Lately concerns have been raised about potential neural ischemia caused by epinephrine acting on epineural vessels and vaso nervorum. This potential risk has to be balanced against lower risk of systemic toxicity, marker for intravascular injection and prolongation of action. • Neal in 2003 adding 5 ug/mL (1:200,000 dilution) prolongs the duration of lidocaine for peripheral nerve blocks from 186 minutes to 264 minutes. Adding only 2.5 ug/mL (1:400,000 dilution) prolongs the block to 240 minutes (almost the same prolongation) without apparent effect on nerve blood flow. Patients with micro angiopathy (e.g., diabetics) who could be at increase risk for neural ischemia secondary to vasoconstriction potentially could benefit from the use of more diluted epinephrine (1:400,000). LOCAL ANESTHETICS ADDITIVES • Opioids • The addition of short-acting opioids such as fentanyl and sufentanil to spinal anesthetics appears to intensify the block and prolong the duration of anesthesia similar to epinephrine without affecting urination. They also prolong analgesia beyond the duration of local anesthetics. When used epidurally they usually produced pruritus. Their usefulness in peripheral nerve blocks is not clear LOCAL ANESTHETICS ADDITIVES • Clonidine • Alpha 2-agonists have analgesic effects when injected on nerves or • • • • in the subarachnoid space. Side effects (hypotension, bradycardia) limit its use but small doses (50-75 ucg) have shown to significantly prolong analgesia in spinal, epidural, intravenous regional, and peripheral nerve blocks both when injected with the local anesthetics and when given orally. Hyaluronidase It breaks down collagen bonds potentially facilitating the spread of local anesthetic through tissue planes. The evidence however shows at least in the epidural space to decrease the quality of anesthesia. Its use seems limited to retrobulbar blocks. Dextran Dextran and other high-molecular-weight compounds have been advocated to increase the duration of local anesthetics. The evidence is lacking. METABOLISM OF LOCAL ANESTHETICS • Ester local anesthetics • They are hydrolyzed at the ester linkage by plasma pseudocholinesterase (also hydrolyses acetylcholine and succinylcholine). The hydrolysis of 2-chloroprocaine is about four times faster than procaine, which in turn is hydrolyzed about four times faster than tetracaine. In individuals with atypical plasma pseudocholinesterase the half-life of these drugs is prolonged and potentially could lead to plasma accumulation. • The hydrolysis of all ester anesthetics leads to the formation of para-aminobenzoic acid (PABA), which is associated with a low potential for allergic reactions. Allergic reactions may also develop from the use of multiple dose vials of amide local anesthetics that contain PABA as a preservative. METABOLISM OF LOCAL ANESTHETICS • Amide local anesthetics • They are transported into the liver before their biotransformation. The two • • major factors controlling the clearance of amide local anesthetics by the liver are: hepatic blood flow and hepatic function. The metabolism of local anesthetics as well as that of many other drugs occurs in the liver by the cytochrome P-450 enzymes. Because the liver has a large capacity for metabolizing drugs it is unlikely that drug interaction would affect the metabolism of local anesthetics. Drugs such as general anesthetics, norepinephrine, cimetidine, propranolol and calcium channel blockers (e.g., diltiazem) can decrease hepatic blood flow and increase the elimination half-life of amides. Similarly decreases in hepatic function caused by a lowering of body temperature, immaturity of the hepatic enzyme system in the fetus, or liver damage (e.g., cirrhosis) lead to a decreased rate of hepatic metabolism of the amides. Renal clearance of unchanged local anesthetics is a minor route of elimination (lidocaine is only 3% to 5% recovered unchanged in the urine of adults while for bupivacaine is 10% to 16%). LOCAL ANESTHETIC TOXICITY • Systemic local anesthesia toxicity is related to plasma levels. Plasma concentration depends on: • The total dose • The net absorption, which depends on: vasoactivity of the drug, site vascularity and use of a vasoconstrictor. • Biotransformation and elimination of the drug from the circulation • Peak local anesthetic blood levels are directly related to the dose administered at any given site. Generally the administration of a 100-mg dose of lidocaine in the epidural or caudal space results in approximately a 1 ucg/mL peak blood level in an average adult. The same dose injected into less vascular areas (e.g., brachial plexus axillary approach or subcutaneous infiltration) produces a peak blood level of app 0.5 ucg/mL. The same dose injected intercostal produces a 1.5 ucg/mL plasma level. LOCAL ANESTHETIC TOXICITY • Systemic local anesthesia toxicity • Peak blood levels may also be affected by the rate of • biotransformation and elimination. In general this is the case only for very actively metabolized drugs such as 2chloroprocaine, which has a plasma half-life of about 45 seconds to1 minute. For amide local anesthetics like lidocaine peak plasma level after regional anesthesia primarily result from absorption. Lidocaine biotransformation half-life is approximately 90 minutes. Local anesthetics interfere with the functions of all organs in which transmission of impulses occurs, among others the CNS and cardiovascular systems. LOCAL ANESTHETIC TOXICITY • Central nervous system • Toxic levels are usually produced by inadvertent intravascular • • • • • • • • • injection. It can also result from the slow absorption following peripheral injection. A sequence of symptoms can include: Numbness of the tongue Lightheadedness Tinnitus Restlessness Tachycardia Convulsions Respiratory arrest LOCAL ANESTHETIC TOXICITY • Cardiovascular system – The cardiovascular manifestations usually follow the CNS effects (therapeutic index). The exception is bupivacaine, which can produce cardiac toxicity at subconvulsant concentrations. – Rhythm and conduction are rarely affected by lidocaine, mepivacaine and tetracaine but bupivacaine and etidocaine can produce ventricular arrhythmias. – EKG shows a prolongation of PR and widening of the QRS – Higher incidence in pregnancy – CV toxicity is increased under hypoxia and acidosis. Treatment of systemic toxicity • ABC (Airway, Breathing and Circulation) is the mainstay • • of treatment. Administration of O2 by mask or bag and mask is often all that is necessary to treat seizures. If seizures interfere with ventilation benzodiazepines, thiopental or propofol can be used. The use of succinylcholine effectively facilitates ventilation and by abolishing muscular activity decreases the severity of acidosis. However neuronal seizure activity is not inhibited and thus cerebral metabolism and oxygen requirements remain increased. . Treatment of systemic toxicity • Little information is available regarding the treatment of cardiovascular toxicity of local anesthetics in humans. Animal data suggest that (1) high doses of epinephrine may be necessary to support heart rate and blood pressure; (2) atropine may be useful for bradycardia; (3) DC cardioversion is often successful; and (4) ventricular arrhythmias are probably better treated with amiodarone than with lidocaine. Amiodarone is used as for ACLS, 150 mg over 10 min, followed by 1 mg/min for 6 hrs then 0.5 mg/min. Supplementary infusion of 150 mg as necessary up to 2 g. For pulseless VT or VF, initial administration is 300 mg rapid infusion in 20-30 mL of saline or dextrose in water. Vasopressin (40 U IV, single dose, one time only) is more frequently used now before epinephrine (1 mg IV every 3-5 minutes). The best treatment for toxic reactions is prevention Maximum dose • Regional anesthesiologists perform peripheral nerve blocks with an amount • • • • of local anesthetic that usually exceeds the maximum recommended doses. The common recommendations for maximum doses as suggested by the literature “are not evidence based” (14) and have proven to be “poor approximation of safety” (15). Many practitioners have called to review these guidelines to better reflect the reality of clinical practice. The American Society of Regional Anesthesia convened a “Conference in Local Anesthetic Toxicity” with a panel of experts in 2001 to discuss the subject. Many papers related to that conference have been published. In a review article by Rosenberg et al (14) the authors propose that the safe ranges should be block specific and related to patient’s age (e.g., epidural), organ dysfunction (especially for repeated doses) and pregnancy. They suggest also adding epinephrine 2.5 to 5 µg/ml when not contraindicated. The fact is that most of the systemic toxicity occurs with unintentional direct intravascular injection Methgemoglobinemia • Prilocaine and benzocaine can oxidize the ferric form of the hemoglobin to the ferrous form, creating methemoglobin. When this exceeds 4 g/dL cyanosis can occur. Depending on the degree Methemoglobinemia can lead to tissue hypoxia. The oxyHb curve shifts to the left (P50 < 27 mmHg). MetHb has a larger absorbance than Hb and 02Hb at 940 nm but simulates Hb at 660 nm. Therefore at high SaO2 levels (more than 85%) the reading underestimates the true value of it or overestimates the O2Hb. At low SaO2 (<85%) the value is falsely high. In the presence of high MetHb concentrations the SaO2 approaches 85% independent of the actual arterial oxygenation. • Methemoglobinemia is easily treated by the administration of methylene blue (1-5mg/kg) or less successfully of ascorbic acid (2 mg/kg). • Allergy • True allergy to local anesthetics is rare. It is relatively more frequent with esters, which are metabolized to para-amino-benzoic acid (PABA). PABA is frequently used in the pharmaceutical and cosmetic industries. Allergy to amide local anesthetics is exceedingly rare. There is no cross allergy between esters and amides. However use of methylparaben as a preservative in multidose vials of lidocaine can elicit allergy in patients allergic to PABA Procaine • Ester pka 8.9 slow onset very short half life (20 sec) protein binding 5% • duration: short 2-chloroprocaine • Ester pka 9.0 rapid onset short duration (it has 30 minutes 2-segment regression in epidural) • serious neurological deficits have occurred after massive intrathecal injection planned for spinal possible associated with the antioxidant bisulfite. The next preservative used ethylenediamine tetraacetic acid (EDTA) was associated with severe muscle spasm after epidural in ambulatory patients. The present solution is prepared without preservative and no back spasms have been reported Tetracaine • Ester pka 8.6 slow onset short plasma half life (2.5 to 4 min) and long duration of action Cocaine • ester pka 8.5 slow onset short duration vasoconstrictor interferes with the reuptake of cathecolamines resulting in hypertension, tachycardia, arrhythmia and myocardial ischemia. Can potentiate cathecolamine-induced arrhythmia by halothane, theophylline or antidepressants Benzocaine • ester (only secondary amine). It limits its ability to pass through membranes. pka 3.5 slow onset short duration Topical anesthetic excessive use is associated with Methemoglobinemia • Lidocaine amide pka 7.7 intermediate onset and duration half-life 45-60 min • Mepivacaine amide pka 7.6 intermediate onset and duration • Bupivacaine amide pka 8.1 Slow onset, long duration Cardiac arrest associated with bupivacaine is difficult to treat possibly due to its high protein binding and high lipid solubility • Ropivacaine amide pka 8.2 chemical analog of mepivacaine and bupivacaine Prepared as L enantiomer Onset and duration as well as potency similar to bupivacaine Cardiac toxicity higher than mepivacaine but lower than bupivacaine • Levobupivacaine amide L enantiomer of bupivacaine similar to ropivacaine