Bonding Review
advertisement

• Ionic bonding
• Covalency
– evidence for ionic
bonding, electron
density maps
– trends in radii
– Born Haber cycles
• explaining formulae,
why AlO is incorrect
– polarisation
• Metallic Bonding
–
–
–
–
electron density maps
giant atomic structures
dot-cross diagrams
shapes of molecules
• VSEPR
– electronegativity
– polarity of covalent
bonds
– polarity of molecules
• Intermolecular forces
– trends in physical properties
• Solutions and dissolving
– why certain substances dissolve in particular solvents
Melting Points of Period 3 elements
2000
Temperature /K
Si
1500
1000
Mg
Al
500
Na
P
S
Cl
0
Ar
Melting Points of Period 2 elements
Temperature / K
4000
C
3000
B
2000
Be
1000
Li
0
N
O
F
Ne
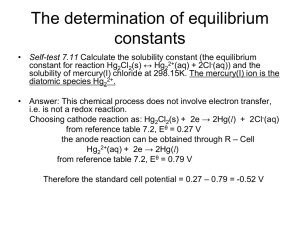
Ionic bonding
• The electrostatic attraction between
oppositely charged ions
– Metals, hydrogen and ammonium form positive
ions (cations).
– Non-metals form negative ions (anions).
Evidence for ionic compounds
• High melting points
– strong electrostatic attractions between oppositely
charged ions
• Electrical conductivity only in liquid state or
aqueous solution because ions need to move.
• Coloured ions can be observed migrating to
electrodes during electrolysis (e.g. CuCr2O7)
– green / blue Cu2+ (aq) moves to cathode
– yellow Cr2O72- (aq) moves to anode
• Electron density maps show low electron density
between the oppositely charged ions.
Na+ (g) + e- + Cl (g)
DHat [Cl]= + 121.7 kJ mol-1
Na+ (g) + e- + ½ Cl2 (g)
Eaff[Cl] = - 348.8 kJ mol-1
Enthalpy (H)
Na+ (g) + Cl- (g)
Em1[Na] = + 496 kJ mol-1
Na (g) + ½ Cl2 (g)
Born Haber cycle for
sodium chloride
DHlatt
DHat = + 107.3 kJ mol-1
Na (s) + ½ Cl2 (g)
(=348.8-121.7-496-107.3-411.2)
= - 787.4 kJ mol-1
DHformation = - 411.2 kJ mol-1
NaCl (s)
K+ (g) + e- + Cl (g)
DHat [Cl]= + 121.7 kJ mol-1
K+ (g) + e- + ½ Cl2 (g)
Eaff[Cl] = - 348.8 kJ mol-1
Enthalpy (H)
K+ (g) + Cl- (g)
Em1[K] = + 419 kJ mol-1
Born Haber cycle for
potassium chloride
K (g) + ½ Cl2 (g)
DHlatt
DHat = + 89.2 kJ mol-1
K (s) + ½ Cl2 (g)
(=348.8-121.7-419-89.2-436.7)
= - 717.8 kJ mol-1
DHformation = - 436.7 kJ mol-1
KCl (s)
K+ (g) + e- + Br (g)
DHat [Br]= + 111.9 kJ mol-1
K+ (g) + e- + ½ Br2 (l)
Eaff[Br] = - 324.6 kJ mol-1
Enthalpy (H)
K+ (g) + Br - (g)
Em1[K] = + 419 kJ mol-1
Born Haber cycle for
potassium bromide
K (g) + ½ Br2 (l)
DHat [K]= + 89.2 kJ mol-1
K (s) + ½ Br2 (l)
DHformation = - 393.8 kJ mol-1
KBr (s)
DHlatt
=(324.6-111.9-419-89.2-393.8)
= - 689.3 kJ mol-1
2 Li+ (g) + O2- (g)
2 Li+ (g) + 2 e- + O (g)
Enthalpy (H)
DHat [O]= + 249.2 kJ mol-1
Eaff[O-]
Eaff[O]=
+798 kJ mol-1
- 141.1 kJ mol-1
2 Li+ (g) + e- + O- (g)
2 Li+ (g) + 2 e- + ½ O2 (g)
DHlatt
(=-798+141.1-249.2-1040-318.8-597.6)
2 Em1[Li] = + 1040 kJ mol-1
= - 2862.8 kJ mol-1
2 Li (g) + ½ O2 (g)
2 DHat = + 318.8 kJ mol-1
2 Li (s) + ½ O2 (g)
DHformation = - 597.9 kJ mol-1
Li2O (s)
Born Haber cycle for
lithium oxide
Mg2+ (g) + 2e- + 2 Cl (g)
Enthalpy (H)
2 DHat [Cl]= + 243.4 kJ mol-1
Mg2+ (g) + 2e- + Cl2 (g)
Em2[Mg] = + 1451 kJ mol-1
Mg+ (g) + e- + Cl2 (g)
Em1[Mg] = + 738 kJ
mol-1
2 Eaff [Cl]
= - 697.6 kJ mol-1
Mg2+ (g) + 2 Cl- (g)
DHlatt =
= - 2526 kJ mol-1
Mg (g) + Cl2 (g)
DHat(Mg) = + 148 kJ mol-1
Mg (s) + Cl2 (g)
DHformation =+148+738+1451+243.4-679.6-2526
- 625.2 kJ mol-1
MgCl2 (s)
Born Haber cycle for
magnesium chloride
Mg+ (g) + e- + Cl (g)
DHat [Cl]= + 121.7 kJ mol-1
Mg+ (g) + e- + ½ Cl2 (g)
Eaff[Cl] = - 348.8 kJ mol-1
Enthalpy (H)
Mg+ (g) + Cl- (g)
Em1[Mg] = + 738 kJ mol-1
Born Haber cycle for
MgCl
Mg (g) + ½ Cl2 (g)
DHat = + 148 kJ mol-1
Mg (s) + ½ Cl2 (g)
DHformation[MgCl (s)]
(=+148+738+121.7-248.8-780)
= - 21.1 kJ mol-1
MgCl (s)
DHlatt[MgCl (s)]
= - 780 kJ mol-1
Enthalpy (H)
Mg3+ (g) + 3e- + 3 Cl (g)
3 DHat [1/2Cl2]= + 366 kJ mol-1
Mg3+ (g) + 3e- + 3/2 Cl2 (g)
Em1+ Em2+Em3 [Mg]
= (738+1451+7738) kJ mol-1
= + 9927 kJ mol-1
Mg (g) + 3/2 Cl2 (g)
DHat = + 148 kJ mol-1
Mg (s) + 3/2 Cl2 (g)
3 Eaff[Cl] = - 1047 kJ
mol-1
Mg3+ (g) + 3 Cl- (g)
Born Haber cycle for
MgCl3
DHlatt = - 4500 kJ mol-1
MgCl3 (s)
DHformation =
+148+9927+366-1047-4500
+ 4894 kJ mol-1
=
Ca2+ (g) + 2e- + 2 I (g)
Enthalpy (H)
2 DHat [I]= + 214 kJ mol-1
2 Eaff[I]= - 590.8 kJ mol-1
Ca2+ (g) + 2e- + I2 (g)
Em2[Ca] = + 1145 kJ mol-1
Ca+ (g) + e- + I2 (g)
Em1[Ca] = + 590 kJ mol-1
Ca (g) + I2 (g)
Ca2+ (g) + 2 I- (g)
Born Haber cycle for
Calcium iodide
DHat [Ca] = + 178.2 kJ mol-1
Ca (s) + I2 (g)
DHlatt
DHformation = - 533.5 kJ
= - 2069.9 kJ mol-1
mol-1
CaI2 (s)
=(590.8-214-1145-590-178.2-533.5)
Mg2+ (g) + O2- (g)
Mg2+ (g) + 2 e- + O (g)
Enthalpy (H)
DHat [O]= + 249.2 kJ mol-1
Eaff[O-]
Eaff[O]=
+798 kJ mol-1
- 141.1 kJ mol-1
Mg2+ (g) + e- + O- (g)
Mg2+ (g) + 2 e- + ½ O2 (g)
Em2[Mg] = + 1451 kJ mol-1
Mg+ (g) + e- + ½ O2 (g)
Em1[Mg] = + 738 kJ mol-1
DHlatt =
(-798+141.1-249.2-1451-738-147.7-601.7)
= - 3844.5 kJ mol-1
Mg (g) + ½ O2 (g)
DHat(Mg) = + 147.7 kJ mol-1
Mg (s) + ½ O2 (g)
DHformation = - 601.7 kJ mol-1
MgO (s)
Born Haber cycle for
magnesium oxide
B3+ (g) + 3e- + 3 F (g)
3 DHat [F]= + 237 kJ mol-1
B3+ (g) + 3e- + 3/2 F2 (g)
3 Eaff[F] = - 984 kJ mol-1
Enthalpy (H)
B3+ (g) + 3 F- (g)
Em1+ Em2+Em3 [B]
= (578+1817+2745) kJ mol-1
= + 5140 kJ mol-1
B (g) + 3/2 F2 (g)
Born Haber cycle for
Boron fluoride
DHlatt
DHat = + 326.4 kJ mol-1
B (s) + 3/2 F2 (g)
(984-237-5140-326.4-1504.1)
= - 6223.5 kJ mol-1
DHformation = - 1504.1 kJ mol-1
BF3 (s)
2 Al3+ (g) + 3 O2- (g)
2 Al3+ (g) + 6 e- + 3 O (g)
Enthalpy (H)
3 DHat [O]
= + 747.6 kJ mol-1
3 Eaff[O-]
3 Eaff[O]=
+2394 kJ mol-1
- 423.3 kJ mol-1
2 Al3+(g)+3e-+3O- (g)
2 Al3+ (g) + 3 e- + 3/2 O2 (g)
2(Em1+ Em2+ Em3 )[Al]
= 2(578+1817+2745) kJ mol-1
= + 10 280 kJ mol-1
DHlatt =
(-2394+423.3-747.6-10280-652.8-1675.7)
= - 15 327 kJ mol-1
2 Al (g) + 3/2 O2 (g)
2 DHat(Al) = + 652.8 kJ mol-1
2 Al (s) + 3/2 O2 (g)
DHformation = - 1675.7 kJ mol-1
Al2O3 (s)
Born Haber cycle for
aluminium oxide
2 B3+ (g) + 3 O2- (g)
2 B3+ (g) + 6 e- + 3 O (g)
Enthalpy (H)
3 DHat [½ O2 (g)]
= + 747.6 kJ mol-1
3 Eaff[O-]
3 Eaff[O]=
+2394 kJ mol-1
- 423.3 kJ mol-1
2 B3+(g)+3e-+3O- (g)
2 B3+ (g) + 3 e- + 3/2 O2 (g)
2(Em1+ Em2+ Em3 )[B]
= 2(801+2427+3660) kJ mol-1
= + 13 776 kJ mol-1
DHlatt =
(-2394+423.3-747.6-13776-1025.4-1273)
= - 18 800 kJ mol-1
2 B (g) + 3/2 O2 (g)
2 DHat(B) = + 1025.4 kJ mol-1
2 B (s) + 3/2 O2 (g)
DHformation = - 1273 kJ mol-1
B2O3 (s)
Born Haber cycle for
boron oxide
Lattice energies
LiF
-1031 LiCl
-848
BeO
-4443
BeCl2
-3020
NaF -918
NaCl
-780
MgO -3791
MgCl2 -2526
KF
KCl
-711
CaO
-3401
CaCl2
-2258
RbF -783
RbCl
-685
SrO
-3223
SrCl2
-2156
CsF
CsCl
-661
BaO
-3054
BaCl2
-2056
-817
-747
AlO is not the formula of aluminium oxide
• Not Al2+ O2• Whilst successive ionisation energies of Al increase, Em3
is not especially large.
• Al3+ is very much smaller than Al2+, since its 3rd
principal quantum shell is now empty.
• Consequently, the ions pack more tightly in (Al3+)2(O2-)3.
• Al3+ also carries a greater charge than Al2+,, increasing
the attraction to O2- anions.
• The lattice energy of (Al3+)2(O2-)3 is therefore much
greater in magnitude than that of Al2+O2-.
AlO is not the formula of aluminium oxide
• Not Al3+ O3• O3- would have a greater radius than O2- since its extra
electron occupies a new principal quantum shell, further
from the nucleus and more shielded by inner quantum
shells.
• In spite of the increased charge of the O3- anion, its large
size reduces packing density of the solid.
• The electron affinity required to form O3- from O2- would
be large and endothermic.
• Therefore the lattice energy of Al3+O3- does not make up
for the endothermic steps in the Born Haber cycle.
Polarisation of the anion
X+
X+
Y-
Y-
Factors leading to anion polarisation
• Cation polarizing power increases with
– small radius (increasing charge density)
– large positive charge (increasing charge density)
• Anion polarizability increases with
– large radius (outer electrons far from nucleus and
shielded by inner shells)
• increasing negative charge increases its size
• Increasing anion polarisation means increasing
covalent character to the bonding
– indicated by large difference between theoretical and
experimental lattice energies
Metallic bonding
• The attraction between 'positive ions' and a
sea of delocalised electrons.
• Why does the melting point increase across
a period, Na<Mg<Al?
• Electrical and thermal conductivity due to
transfer of charge and energy by the
movement of delocalised electrons.
The covalent bond
• The attraction between two nuclei and a shared
pair of electrons.
• One electron of the shared pair originating from
each atom in a 'standard' covalent bond.
• Both electrons of the shared pair originate from the
same atom in a dative bond.
• 'Standard' and dative covalent bonds are
indistinguishable.
Hydrogen molecule (H2)
H
H
H-H
Valence Shell Electron Pair Repulsion
• Sigma bond electron pairs and lone pairs all repel
each other around the central atom.
• The electron pairs move into positions of maximum
separation.
– 2 pairs gives 180: 3 pairs gives 120: 4 pairs gives
109.5, 6 pairs gives 90.
• Lone pairs have a greater repulsion than sigma bond
pairs.
– Each lone pair reduces the expected bond pair - bond pair
angle by about 2.5.
Chlorine molecule, Cl2
Cl
Cl
Cl-Cl
Hydrogen chloride molecule, HCl (g)
{not HCl (aq) which is ionic}
H
Cl
Why is this covalent?
H-Cl
Water molecule, H2O
H
O
H
O
H
H
109.5
Nitrogen molecule, N2
N
N
N
N
Ammonia, NH3
H
N
H
H
N
H H H
107
Methane, CH4
H
H
C
H
H
H
C
H
109.5
H
H
H
H C H
H
Ethane, C2H6
H
H3C
H
C
H
C
H
H
C
H
H
109.5
H
H H
H C C H
H H
H
Ethene, C2H4
H
121
H
H
C=C
H
C
H
118
H
C
H
H
Carbon dioxide, CO2
O
O=C=O
C
O
H2S
H
H
S
H
S H
104
SiH4
H
H
H
Si
H
H Si H
109.5
H
H
Methanal, HCHO
O
H
C
H
C
120
H
O
H
120
HCN
H
C
N
H C N
180
H
H
O
O
H
104
O O
104
H
+
H
H
H
N
H
+
H N H
109.5
H
H
O
H
2O
O
O
O
S
O
2-
O
S
109.5
O
O
2O
O
S
S
S
O
2-
O
S
109
O
O
Ethene
H
H
C
C
H
H
• 3 s-bond pairs
around each C atom
repel to positions of
maximum
separation.
• trigonal planar
H
121
H
118
C=C
H
H
Ethene
H
H
H
C
or
C
H
H
C
C
H
H
H
H
H
H
C
C
H
H
C
C
H
H
H
s bonds shown as
lines and wedges
Benzene, C6H6
neither
nor
but
Benzene
• p bonding is delocalised over the whole
ring because all 6 p orbitals are coplanar
and overlap.
– not 3 separate p bonds
• Benzene is more stable than alkenes and
tends to react by substitution rather than
addition.
Pyrene, C16H10
Graphite
Flat sheet of
C atoms
Weak
forces
between
sheets
How to draw diamond
Diamond
The ice structure
Dissolving an ionic solid
H
O
H
H
O
H
+ (aq)
H
+ O
H
Dissolving
• Forces are broken between the particles
within the solute and within the solvent
• The energy required to break these forces
needs to come from new attractive
interactions between solvent and solute
particles.
– If this energy is not supplied, the ‘solute’ does
not dissolve.
Dissolving (2)
• Non-polar molecules attract by London
forces alone.
• Ionic solutes have strong electrostatic
attractions between the ions.
– The energy required to overcome the high
lattice energy can often be supplied through
hydration of the ions by the highly polar water
molecule
– Water can also hydrogen bond to polar solutes
such as sugars and ethanol.
Dissolving (3)
• Water does not dissolve non-polar solutes.
– Hydrogen bonds would need to be broken between
solvent water molecules.
– This energy is not made up for by London forces
between solvent and solute molecules.
• Non-polar solvents dissolve non-polar solutes.
– Little energy is required to break London forces between
the solvent molecules.
– This energy is supplied by new London forces between
solute and solvent molecules.
Dissolving (4)
• Metals dissolve other metals
• Ionic solids (such as alumina) dissolve in
ionic solvents (such as cryolite, Na3AlF6)