notes
advertisement

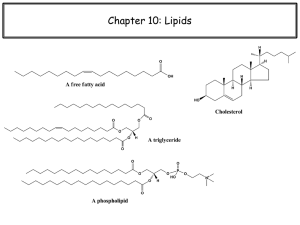
Lipids Class Presentations Will be Discussed at the End of Class Exams back next Monday No class this Wednesday !!!!! Lipids Main functions of lipids in foods Energy and maintain human health Influence on food flavor Fatty acids impart flavor Lipids carry flavors/nutrients Influence on Solids food texture or liquids at room temperature Change with changing temperature Participation in emulsions Lipids Lipids are soluble in many organic solvents Ethers (n-alkanes) Alcohols Benzene DMSO (dimethyl sulfoxide) They are generally NOT soluble in water C, H, O and sometimes P, N, S Lipids Neutral Lipids Waxes Long-chain alcohols (20+ carbons in length) Cholesterol esters Vitamin A esters Vitamin D esters Conjugated Lipids Triacylglycerols Phospholipids, glycolipids, sulfolipids “Derived” Lipids Fatty acids, fatty alcohols/aldehydes, hydrocarbons Fat-soluble vitamins Lipids Structure Triglycerides or triacylglycerols Glycerol + 3 fatty acids >20 different fatty acids Lipids 101 Fatty acids- the building block of fats A fat with no double bonds in it’s structure is said to be “saturated” (with hydrogen) Fats with double bonds are referred to as mono-, di-, or tri- Unsaturated, referring to the number of double bonds. Some fish oils may have 4 or 5 double bonds (polyunsat). Fats are named based on carbon number and number of double bonds (16:0, 16:1, 18:2 etc) Lipids liquid triacylglycerides “Oleins” Fat- solid or semi-solid mixtures of crystalline and liquid TAG’s “Stearins” Lipid content, physical properties, and preservation are all highly important areas for food research, analysis, and product development. Many preservation and packaging schemes are aimed at prevention of lipid oxidation. Oil- Nomenclature The first letter C represents Carbon The number after C and before the colon indicates the Number of Carbons The letter after the colon shows the Number of Double Bonds ·The letter n (or w) and the last number indicate the Position of the Double Bonds Saturated Fatty Acids Saturated Fatty Acids 8 7 CH3 CH2 O 5 3 4 6 2 1 CH2 CH2 CH2 CH2 CH2 C Octanoic Acid OH Mono-Unsaturated Fatty Acids Poly-Unsaturated Fatty Acids Fatty Acids Melting Points and Solubility in Water Melting Point z Solubility in H2O 2 Fatty acid chain length Unsaturated Fatty Acids 8 CH3 7 CH2 5 6 CH2 CH2 4 CH2 O 3 2 1 CH2 CH2 C OH 3 - Octenoic Acid 8 7 CH3 CH2 O 5 3 4 6 2 1 CH2 CH2 CH2 CH2 CH2 C 3, 6 - Octadienoic Acid OH Lipids Properties depend on structure Length of fatty acids (# of carbons) Position of fatty acids (1st, 2nd, 3rd) Degree of unsaturation: Double bonds tend to make them a liquid oil Hydrogenation: tends to make a solid fat Significantly lowers the melting point Significantly increases the melting point Unsaturated fats oxidize faster Preventing lipid oxidation is a constant battle in the food industry Fatty Acids Melting Points and Solubility in Water Melting Point z Solubility in H2O 2 Fatty acid chain length Characteristics of Fatty Acids Fatty Acids M.P.(C) mg/100 ml in H2O C4 -8 C6 -4 970 C8 16 75 C10 31 6 C12 44 0.55 C14 54 0.18 C16 63 0.08 C18 70 0.04 Fatty Acids O R C OH #1 Carbon O R C OH Acid Group Polar End - Hydrophilic End Non-polar End - Hydrophobic End (Fat-soluble tail) Lipids 101 Fatty acid profile- quantitative determination of the amount and type of fatty acids present following hydrolysis. To help orient ourselves, we start counting the number of carbons starting with “1” at the carboxylic acid end. O C C C C C C C C C C C C C C C C C C 18 1 OH Lipids 101 For the “18-series” (18:0, 18:1, 18:2, 18:3) the double bonds are usually located between carbons 9=10 12=13 15=16. O C C C C C C C C C C C C C C C C C C 18 16 15 13 12 10 9 1 OH Lipids 101 The biomedical field started using the OMEGA (w) system (or “n” fatty acids). With this system, you count just the opposite. Begin counting with the methyl end Now the 15=16 double bond is a 3=4 double bond or as the medical folks call it….an w-3 fatty acid C C C C C C C C C C C C C C C C C C C 1 6 7 3 4 18 9 10 OH Tuning Fork Analogy-TAG’s Envision a Triacylglyceride as a loosely-jointed E Now, pick up the compound by the middle chain, allowing the bottom chain to hang downward in a straight line. The top chain will then curve forward and form an h Thus the “tuning fork” shape Fats will tilt and twist to the lowest free energy level Lipids Lipids are categorized into two broad classes. The first, simple lipids, upon hydrolysis, yield up to two types of primary products, i.e., a glycerol molecule and fatty acid(s). The other, complex lipids, yields three or more primary hydrolysis products. Most complex lipids are either glycerophospholipids, or simply phospholipids contain a polar phosphorus moiety and a glycerol backbone or glycolipids, which contain a polar carbohydrate moiety instead of phosphorus. Lipids Other types of lipids Phospholipids Structure similar to triacylglycerol High in vegetable oil Egg yolks Act as emulsifiers Where Do We Get Fats and Oils? “Crude” fats and oils are derived from plant and animal sources Several commercial processes exist to extract food grade oils Most can not be used without first “refining” before they reach consumers During oil refining, water, carbohydrates, proteins, pigments, phospholipids, and free fatty acids are removed. Crude fats and oils can therefore be converted into high quality edible oils In general, fat and oil undergo four processing steps: Extraction Neutralization Bleaching Deodorization Oilseeds, nuts, olives, beef tallow, fish skins, etc. Rendering, mechanical pressing, and solvent extraction. Fats and Oils: Processing Extraction Rendering Pressing oilseeds Solvent extraction Soybean Peanut Rape Seed Safflower Sesame Fats and Oils Further Processing Degumming Refining Remove free fatty acids (alkali + water) Bleaching Remove phospholipids with water Remove pigments (charcoal filters) Deodorization Remove off-odors (steam, vacuum) Where Do We Get Fats and Oils? Rendering Primarily for extracting oils from animal tissues. Oil-bearing tissues are chopped into small pieces and boiled in water. The oil floats to the surface of the water and skimmed. Water, carbohydrates, proteins, and phospholipids remain in the aqueous phase and are removed from the oil. Degumming may be performed to remove excess phospholipids. Remaining proteins are often used as animal feeds or fertilizers. Where Do We Get Fats and Oils? Mechanical Pressing Mechanical pressing is often used to extract oil from seeds and nuts with oil >50%. Prior to pressing, seed kernels or meats are ground into small sized to rupture cellular structures. The coarse meal is then heated (optional) and pressed in hydraulic or screw presses to extract the oil. Olive oils is commonly cold pressed to get extra virgin or virgin olive oil. It contains the least amount of impurities and is often edible without further processing. Some oilseeds are first pressed or placed into a screwpress to remove a large proportion of the oil before solvent extraction. Where Do We Get Fats and Oils? Solvent Extraction Organic solvents such as petroleum ether, hexane, and 2-propanol can be added to ground or flaked oilseeds to recover oil. The solvent is separated from the meal, and evaporated from the oil. Neutralization Free fatty acids, phospholipids, pigments, and waxes exist in the crude oil These promote lipid oxidation and off-flavors (in due time) Removed by heating fats and adding caustic soda (sodium hydroxide) or soda ash (sodium carbonate). Impurities settle to the bottom and are drawn off. The refined oils are lighter in color, less viscous, and more susceptible to oxidation (without protection). Bleaching The removal of colored materials in the oil. Heated oil can be treated with diatomaceous earth, activated carbon, or activated clays. Colored impurities include chlorophyll and carotenoids Bleaching can promote lipid oxidation since some natural antioxidants are removed. Where Do We Get Fats and Oils? Deodorization The final step in the refining of oils. Steam distillation under reduced pressure (vacuum). Conducted at high temperatures of 235 - 250ºC. Volatile compounds with undesirable odors and tastes can be removed. The resultant oil is referred to as "refined" and is ready to be consumed. About 0.01% citric acid may be added to inactivate prooxidant metals. Fats and Oils Further Processing Hydrogenation Add hydrogen to an oil to “saturate” the fatty acid double bonds Conducted with heated oil Often under pressure In the presence of a catalyst (usually nickel) Converts liquid oils to solid fats Raises melting point Hydrogenating Vegetable oils can produce trans-fats H H C C Cis- H C C Trans- H The cis- and trans- forms of a fatty acid Fats and Oils in Foods SOLID FATS are made up of microscopic fat crystals. Many fats are considered semi-solid, or “plastic”. PLASTICITY is a term to describe a fat’s softness or the temperature range over which it remains a solid. Even a fat that appears liquid at room temperature contains a small number of microscopic solid fat crystals suspended in the oil…..and vice versa PLASTIC FATS are a 2 phase system: Plasticity is a result of the ratio of solid to liquid components. Solid phase (the fat crystals) Liquid phase (the oil surrounding the crystals). Plasticity ratio = volume of crystals / volume of oil Measured by a ‘solid fat index’ or amount of solid fat or liquid oil in a lipid As the temperature of a plastic fat increases the fat crystals melt and the fat will soften and eventually turn to a liquid. Fat and Oil: Further Processing Winterizing (oil) Cooling a lipid to precipitate solid fat crystals DIFFERENT from hydrogenation Plasticizing Modifying (fat) fats by melting (heating) and solidifying (cooling) Tempering (fat) Holding the fat at a low temperature for several hours to several days to alter fat crystal properties (Fat will hold more air, emulsify better, and have a more consistent melting point) Lipid Oxidation Oleic acid Radical Damage, Hydrogen Abstraction Formation of a Peroxyl Radical Simplified scheme of lipoxidation H H H H H H H H H H H H R C C C C R R C C C C R R C C C C R H H + Catalyst H * + Oxygen H O O Primary Drivers Temperature-basic Water rxn kinetics Activity Both high and low Aw At low Aw, peroxides decompose faster and metal ions are better catalysts in a dry environment Metal Ions-catalysts Light-energy source Singlet Oxygen- ROS, highly electrophilic Reacts 1,500 times faster at C=C than ground state O2 Enzymes ie. Lipoxygenase (LOX) Initiation of Lipid Oxidation There must be a catalytic event that causes the initiation of the oxidative process Enzyme catalyzed “Auto-oxidation” Excited oxygen states (i.e singlet oxygen): 1O2 Triplet oxygen (ground state) has 2 unpaired electrons in the same spin in different orbitals. Singlet oxygen (excited state) has 2 unpaired electrons of opposite spin in the same orbital. Metal ion induced (iron, copper, etc) Light Heat Free radicals Pro-oxidants Chlorophyll Water activity Considerations for Lipid Oxidation Which hydrogen will be lost from an unsaturated fatty acid? The longer the chain and the more double bonds….the lower the energy needed. Bond Strength 85 kCal 103 kCal 65 kCal 65 kCal Fatty acid .OH or other Free radicals .. . Propagation Reactions Initiation Peroxyl radical Ground state oxygen Hydroperoxide decomposition Hydroperoxide Alkoxyl radical Start all over again… New Radical Hydroxyl radical!! Mechanism of Photooxidation Chlorophyll 3O 1 or 2 Singlet Oxygen Oxidation 1 O2 HOOC OOH HOOC OOH HOOC OOH HOOC OOH HOOC Autoxidation 9 HOOC 10 12 13 11 8 14 H H 9 10 12 + 13 11 8 9 10 12 14 13 10 9 11 + 8 12 13 11 14 8 14 O2 OO 10 9 8 12 13 11 14 Singlet Oxygen Oxidation 1 O2 HOOC HOOC OOH OH O Nonanal Secondary Products: Aldehydes Volatile Aldehydes from Oxidation Lipid Oxidation Peroxide decomposition can generate aldehydes, ketones, alcohols, various hydrocarbons, and epoxides. Many are volatile, and many are unappealing. Relative Oxidation Rates How fast is lipid oxidation? Fatty Acid Relative Rate Steric acid (18:0) 1 Oleic acid (18:1 n9) 100 Linoleic acid (18:2 n6) 1,200 Linolenic acid (18:3 n3) 2,500 Termination of Lipid Oxidation Although radicals can “meet” and terminate propagation by sharing electrons…. The presence or addition of antioxidants is the best way in a food system. Antioxidants can donate an electron without becoming a free radical itself. Antioxidants and Lipid Oxidation BHT – butylated hydroxytoluene BHA – butylated hydroxyanisole TBHQ – tertiary butylhydroquinone Propyl gallate Tocopherol – vitamin E NDGA – nordihydroguaiaretic acid Carotenoids Polar Antioxidants Most effective in nonpolar or less polar environment Bulk oils Located at the oil-air interface or in reverse micelles High amount of oxidants present here Yellow Oil Blue water Phospholipids Non-polar Antioxidants Most effective in polar environment Oil-in-water emulsions Located at the water-oil interface Dissolved in oil droplets of the emulsion Allows access to oxidizing agents located in the water phase Peroxides Oxidizing metals Chemical Tests for Lipid Characterizations Iodine Value Measure of the degree of unsaturation in an oil or the number of double bonds in relation to the amount of lipid present Defined as the grams of iodine absorbed per 100-g of sample. The higher the amount of unsaturation, the more iodine is absorbed. Therefore the higher the iodine value, the greater the degree of unsaturation. Iodine Value A known solution of KI is used to reduce excess ICl (or IBr) to free iodine R-C-C = C-C-R + ICl R-C-CI - CCl-C-R + ICl [Excess] (remaining) scheme: ICl + 2KI KCl + KI + I2 The liberated iodine is then titrated with a standardized solution of sodium thiosulfate using a starch indicator I2 + Starch + thiosulfate = colorless endpoint Reaction (Blue colored) Iodine Value Used to characterize oils: Following hydrogenation During oil refining (edible oils) Degree of oxidation (unsaturation decreases during oxidation) Comparison of oils Quality control Iodine value: g absorbed I2/ 100 g fat Iodine Value of some oils (Table 14-2) Source Beef Tallow Olive, Palm, Peanut Corn, Cottonseed I2 Value <50 < 100 100 - 130 Highly saturated High in 18:1 High in 18:1 and 18:2) Linseed, Soybean, safflower, conola > 130 18:1, 18:2, 18:3 Fish >150 18:1, 18:2, 18:3 (longer chains) What can we conclude about the COMPOSITION or STRUCTURE of each of these oil types? Automated Iodine Value Determination Standard Iodine Value A = 23 B = 44 C = 67 D = 89 E = 111 Measures IBr or ICl Consumption (neg. peak) Consumption over time Chemical Tests Saponification Value Saponification Value Saponification is the process of breaking down or degrading a neutral fat into glycerol and fatty acids by treating the sample with alkali. Heat Triacylglyceride ---> Fatty acids + Glycerol KOH Definition: mg KOH required to titrate 1g fat (amount of alkali needed to saponify a given amount of fat) Typical values: Peanut = 190, Butterfat = 220 Saponification Value The mg KOH required to saponify triacylglycerides into glycerol plus fatty acids is related to: average fatty acid chain length or average fatty acid molecular weight Divide molecular weight by 3 to get average of the fatty acids present Chemical Tests for Oxidation Lipid Oxidation Hydrolysis Peroxide Value Oxidation Tests LIPID OXIDATION Reactants and Products 35 Lipid System Under Oxidizing Conditions 30 25 Oxygen Uptake 20 Peroxides 15 Secondary Products 10 5 0 1 2 3 4 5 Time 6 7 8 9 Free Fatty Acids (FFA’s) Degree of hydrolysis (hydrolytic rancidity) High level of FFA means a poorly refined fat or fat breakdown after storage or use. Measures of Oxidation Oxidation is a very complex reaction - no one test will measure all of the reactants or products. Some assays measure intermediates while others measure end products. Peroxide Value Measures peroxides and hydroperoxides in an oil which are the primary oxidation products (usually the first things formed). The peroxide value measures the “present status of the oil”. Since peroxides are destroyed by heat and other oxidative reactions, a seriously degraded oil could have a low PV. Peroxide Value KI + peroxyl radical yields free Iodine (I2) The iodine released from the reaction is measured in the same way as an iodine value. I2 in the presence of amylose is blue. I2 is reduced to KI and the endpoint determined by loss of blue color. 4I + O2 + 4H 2I2 + 2H2O Thiobarbituric acid (reactive substances) TBA OR TBARS Tests for end products of oxidation – aldehydes, Malonaldehyde (primary compound), alkenals, and 2,4-dienals A pink pigment is formed and measured at ~530 nm. TBARS is firmly entrenched in meat oxidation research and is a method of choice. TBARS measure compounds that are volatile and may react further with proteins or related compounds. High TBA = High Oxidative Rancidity HEXANAL Determination Good indictor of the end products of oxidation (if there are any). Standard method in many industries. Aldehyde formation from lipid oxidation. Nonenal is also a common end-product Conjugated Fatty Acids During oxidation, double bond migration occurs and conjugated fatty acids are formed. They absorb light efficiently and can be monitored in a spectrophotometer. R C C C C C C C C R TECHNIQUES OF MEASURING OXIDATIVE STABILITY Induction Period: is defined as the length of time before detectable rancidity or time before rapid acceleration of lipid oxidation MEASURING OXIDATIVE STABILITY Active Oxygen Method - Air is bubbled through oil or fat at 97.8°C. Time required to reach peroxide value of 100 meq/kg fat determined. (method replaced by OSI) Stability Index – automated Rancimat (instrumental method). Air bubbled through sample (110°C). Oil degrades to many acidic volatiles (e.g. formic acid) which are carried by the air into a water trap. Conductivity of the water can then be assessed. Oil Free Radicals What are free radicals? Where are free radicals from? How damaging are free radicals? How do we control free radicals? What are free radicals? Any molecular species capable of independent existence, which contains one or more unpaired valence electrons not contributing to intramolecular bonding….is a free radical. The most frequent radicals are oxygen-derived free radicals, also known as reactive oxygen species (ROS): Superoxide (O2·-) Peroxyl (ROO˙) Alkoxyl (RO˙) Hydroxyl (HO˙) Nitric oxide (NO˙) Other ROS are non-radicals such as singlet oxygen (O2), hydrogen peroxide (H2O2), and hypochlorous acid (HClO). Where do they come from? Free radicals are produced by oxidation/reduction reactions in which there is a transfer of only one electron at a time, or when a covalent bond is broken and one electron from each pair remains with each atom. 1) Normal ongoing metabolism, especially from the electron transport system in the mitochondria and from a number of normally functioning enzymes 2) Environmental factors such as pollution, radiation, cigarette smoke and toxins can also spawn biologically-derived free radicals. How damaging are free radicals? ROS may be very damaging, since they can attack: Lipids in cell membranes Proteins in tissues or enzymes Carbohydrates DNA These cause cell membrane damage, protein modification, and DNA damage. Thought to play a role in aging and several degenerative diseases (heart disease, cataracts, cognitive dysfunction, and cancer). Oxidative damage can accumulate with age. Our Body vs. Our Food Biological radicals Food-based radicals Where do these 2 areas cross? Functional Foods Concept Certain food ingredients have health benefits beyond basic nutrition Recent development only: since ~1975 The concept that ‘non-nutrients’ were beneficial has taken off since then First idea in scientific community: antioxidant compounds may protect against chronic diseases Free Radicals Early 1950’s: cell damage is due to reactive oxygen species called “free radicals” Unstable, ‘damaged’ molecule that is missing an electron Highly reactive; reacting to some measurable extent with any molecule they come in contact with In living systems, cell injury or disease In foods, quality-degrading impact Reactive Oxygen Species (ROS) Primary target list: protein, lipid, DNA, and carbohydrates End results: cancer, CHD, stroke, arterial disease, rheumatoid arthritis, Parkinson’s/Alzheimer disease, cataracts, macular degeneration….many more Aging by slow oxidation? The Defense Minimize contact between free radicals and important systems (like cellular components) Cell membranes are one of our best barriers Metal chelation system in-place Protease enzymes are in place to remove damaged proteins for replacement by new “Repair enzymes” help to restore DNA “Antioxidant enzymes”-superoxide dismutase, catalase, glutathione peroxidase Best Defense…A Good Offense “Nutrients” that can’t be synthesized in vivo: vitamin C, vitamin E, (pro)vitamin A “Non-nutrients”: polyphenolics/carotenoids Diet What is only source….are they “essential”? about conditions of “oxidative stress”? This is a condition when pro-oxidants outnumber antioxidants (I.e. decreased immune response, environmental factors, hypertension, poor diet). Foods and the Antioxidant Link Soy- isoflavones, polyphenolics Tea- polyphenolics, flavans Coffee- polyphenolics Wine- polyphenolics Rosemary- carnosic acid, rosmaric acid Citrus- flavonoids Onions- sulfur cpds, flavonoids Berries- flavonoids, polyphenolics Vegetables- carotenoids, polyphenolics Antioxidants in Food Systems Oxidative Stress-the food remedy Diet: Inflammation- tocopherol Smoke- ascorbic acid Physical stress- carotenoids Pollution- carotenoids Environment: Radiation- glutathione Carcinogens- antioxidant enzymes, diet modification Oxidative Stress and Foods Tocopherol- vegetable oil, whole grains, vegetables, fish/poultry Ascorbic acid- citrus, berries, tomato, leafy veggies, brassicas (broccoli, cauliflower) Carotenoids- yellow/orange fruits and veggies, tomatoes, green leafy veggies. Polyphenolics- coffee/tea, grains, all fruits and vegetables Magic Bullets…for our body? Most likely not… Will increasing the intake of antioxidants modulate disease prevention? Will we live longer with no health problems? Lung cancer and ß-carotene: Whoa… Antioxidant compounds have demonstrated benefits (both acute and long-term) of preventing or postponing the onset of many degenerative diseases, but clinical trials are full of holes, and conclusive evidence of “the bullet” is still not with us. Magic Bullets…for our foods? Will increasing the use of antioxidants in foods modulate all oxidative damage? Will food products “live” longer with no quality problems? Pro-oxidant nature of ascorbic acid: Whoa… Ascorbic acid does not always act linearly in food systems In the presence of metal ions (ie. Fe/Cu) it can generate reactive oxygen species (peroxides) or free radicals (hydroxyl radicals) Causes and Effects ß-carotene and lung cancer: small, but significant increase with smokers Tocopherols and CHD: protect lipoproteins or inhibit blood clotting (which initiates heart attacks) Tocopherols and Alzheimer’s: reducing oxidative stress by supplementation Cataracts and vitamins (A,C,E): inverse association Macular degeneration and carotenoids: inverse Vitamin C and the Common Cold: shorter, milder colds Structure-Based AOX Polyphenolics Structure of flavonoids OH 3’ HO 7 6 8 O A 5 C 4 B 2 3 X OH O Flavonols: X=OH Flavones: X=H Flavanones: No 2-3 db 4’ 5’ OH B-Ring Substitutions HO O OH B Kaempferol A C OH OH O Quercetin OH OH HO O Myricetin OH OH OH O OH HO O OH OH OH O Quercetin 4 –OH groups OH OH HO O 2-3 db OH OH O 3-OH 4-oxo function Catechin 4 –OH groups OH OH HO O OH OH 3-OH Cyanidin 4 –OH groups OH OH + HO O OH OH Structurally Similar Compounds OH OH OH OH HO HO O O OH OH OH OH O Quercetin AOX = 4.7 OH OH + O HO Cyanidin OH AOX = 4.4 OH Catechin AOX = 2.4 Importance of the 3-OH group OH OH HO O O O OH OH HO O Quercetin-3-glucoside AOX ~ 2.5 O OH O O OH OH O Quercetin AOX = 4.7 O OH O OH HO O OH O Luteolin AOX = 2.1 Importance of the 4-Oxo Function Works with the 2-3 double bond in the C-ring and is responsible for electron delocalization from the B-ring. 3-OH and 5-OH substitutions with the 4-oxo function are best for maximum AOX properties OH OH HO Structurally, quercetin has all the right components to make for the “perfect” antioxidant. O OH OH O Importance of the 2-3 db OH OH OH OH HO HO O O OH OH OH O Quercetin AOX = 4.7 OH O Taxifolin AOX = 1.9 More on the Phenolic Acids COOH COOH COOH 1 2 Ortho 3 4 Para Meta Hydroxybenzoic Acid (HBA) 1 1 2 2 3 3 4 4 Hydroxyphenylacetic Acid (HPA) Cinnamic Acid (CA) COOH HO 0.08 COOH HO 1.19 p-OH-benzoic p-coumaric Protocatechuic Caffeic HO COOH 2.22 HO COOH 1.26 HO HO MeO MeO HO COOH 1.43 Vanillic Ferulic HO 1.90 COOH Antioxidants in Food Systems What Makes a Good Antioxidant? Polyphenolics- Radical scavengers Number of hydroxy groups (-OH) Location of hydroxy groups (on benzene ring) Presence of a 2-3 double bond (flavylium ring) 4-oxo function (flavylium ring) Synergistic/antagonistic reactions with other antioxidant compounds OH OH HO HO O COOH COOH OH HO OH O What Makes a Good Antioxidant? Carotenoids The number of conjugated double bonds (9+ is best) Substitutions on ß-ionone group (on the end) Radical scavengers Beta-Carotene R* + CAR => R- + CAR*+ Chain breakers ROO*+ CAR => ROO-CAR* ROO-CAR* + ROO* => ROO-CAR-ROO Singlet 1 oxygen quenchers O2* + 1CAR => 3O 2 + 3CAR* Tocopherol Alpha-tocopherol = Vitamin E beta and gamma forms also Synergist with carotenoids and selenium and is regenerated by vitamin C Efficiency determined by the bond dissociation energy of the phenolic -OH bond The heterocyclic chromanol ring is optimized for resonance stabilization of an unpaired electron. HO O Antagonism-Synergism-Metals Many antioxidant work for and against each other An antioxidant in a biological system my be regenerated In mixed ROS…inefficiency of one antioxidant to quench all the different radicals. No way of knowing if the “better” antioxidant for a particular radical is doing all the work or not. Will a better antioxidant for a given food system “beat out” a lesser antioxidant (antagonistic response) in order to quench the radicals. Example: Factors Affecting AOX of Bell Peppers Chemical interactions In vitro models Find synergistic/antagonistic effects Free metal ions Diluted isolates Add metal chelator Flavonoid Ascorbic AOX ? AOX with Quercetin Interactions (ß-Carotene Bleaching) Rate of Inhibition 60 Quercetin Only 50 Quercetin + 5 mM Ascorbic acid 40 Quercetin + 2.5 mM Caffeic acid 30 20 10 0 -10 10 20 30 450 ppm Caffeic = 47 % 880 ppm Ascorbic = 15% 40 50 60 70 Quercetin (ppm) 80 90 100 HO OH OH O OH OH O AOX with Luteolin Interactions (ß-Carotene Bleaching) Rate of Inhibition 90 80 Luteolin only 70 Luteolin + 2.5 mMCaffeic acid 60 Luteolin + 5 mM Ascorbic acid 50 40 30 20 10 0 OH OH -10 10 20 30 450 ppm Caffeic = 47 % 880 ppm Ascorbic = 15% 40 50 60 70 80 HO 90 O Luteolin (ppm) OH O 100 Theoretical Quercetin “Regeneration” Scheme OH O B HO OH O Quercetin Quercetin HO O A C OH OH OH OH HO OH OH O OH O H. O OH O O Reduced resonation in A and B-ring Minor regeneration by ascorbic acid Reduced resonation in A and B rings Minor regeneration by caffeic acid Delocalization of C-ring Delocalization of C-ring Minor regeneration by ascorbic acid Minor regeneration by caffeic acid Theoretical Luteolin “Regeneration” Scheme OH OH OH HO O B OH OH HO O OH HO O A C OH O Luteolin Luteolin H. O O O O Excellent resonance stability in A-ring Excellent resonance stability in A-ring Highly regenerated by ascorbic acid No regeneration by caffeic acid acid Highly regenerated by ascorbic Electron donation by C-ring Electron donation by C-ring No regeneration by caffeic acid Antioxidant Activity after Dilution % Inhibition of Carotene Bleaching 50 Bell Pepper Juice (Boiled) 40 30 20 10 0 -10 -20 -30 1X 2X 4X 8X Dilutions 16X 32X Antioxidant Activity with Chelator % Inhibition of Bleaching 100 Bell Pepper Juice (Boiled) Bell Pepper Juice + 500 ppm EDTA 80 60 40 20 0 -20 100 % Juice 75% Juice 50% Juice 25% Juice Antioxidant Methods HAT and SET Reactions Hydrogen Atom Transfer (HAT) vs. Single Electron Transfer (SET) Antioxidants can work in one of two ways (HAT or SET). End result is the same for both, differing in kinetics and side rxns. HAT and SET rxns may occur in parallel Determined by antioxidant structure and properties Solubility and partition coefficient System solvent, system pH HAT HAT-based methods measure the classical ability of an antioxidant to quench free radicals by hydrogen donation (AH = any H donor) SET SET-based methods detect the ability of a potential antioxidant to transfer one electron to reduce any compound, including metals, carbonyls, and radicals. Also based on deprotonation, so pH dependent HAT vs SET HAT Selectivity in HAT rxs are determined by the bond dissociation energy of the H-donating group in the antioxidant Antioxidant reactivity or capacity measurements are therefore based on competition kinetics. Reactions are solvent and pH independent and are very fast Common reducing agents (Vitamin C) are an interference SET Usually slow and can require long times to reach completion Antioxidant reactivity is based on a percent decrease, rather than kinetics Very sensitive to ascorbic acid and other reducing agents. Trace amounts of metal ions will interfere, and cause overestimation and inconsistent results. Antioxidants and Radicals Four sources of antioxidants: Enzymes Large molecules estrogen, angiotensin, melatonin Multiple free radical and oxidant sources ascorbic acid, glutathione, uric acid, tocopherol, carotenoids, phenols Hormones albumin, ferritin, other proteins Small molecules Superoxide dismutase, glutathione peroxidase, and catalase O2, O2·-, HO˙, NO˙, ONOO-, HOCl, RO(O)˙, LO(O) Oxidants and antioxidants have different chemical and physical characteristics. Complex Systems: Singlet Oxygen Carotenoids are not good peroxyl radical quenchers compared to polyphenolics Carotenoids are exceptional singlet oxygen quenchers compared to polyphenolics However, singlet oxygen is not a radical and does not react via radical mechanisms Singlet oxygen reacts by its addition to fatty acid double bonds, forming endoperoxides, that can be reduced to alkoxyl radicals, that initiate radical chain reactions. Now we have multiple reaction characteristics and multiple mechanisms No single assay will accurately reflect all of the radical sources or test all the antioxidants in such a complex system. Method Selections for Antioxidants Controversy exists over standard methods for antioxidant determination Historical use and peer-review acceptance is critical Use my multiple labs to highlight strength, weakness, and effectivness New methods take time to adopt and accept An “ideal” method: Measures chemistry actually occurring in potential application Utilizes a biologically relevant radical source Simple to run Uses a defined endpoint and chemical mechanism Instrumentation is readily available Good within-run and between-day reproducibility Adaptable for both hydrophilic and lipophilic antioxidants Adaptable for multiple radical sources Adaptable for high-through-put analysis Understanding of the range of use and recognition of interfering agents HAT assays ORAC Oxygen Radical Absorbance Capacity Measures inhibition of peroxyl radical induced oxidations in chain breaking activity by H atom transfer TRAP Total Radical-Trapping Antioxidant Parameter Measures the ability to interfere with peroxyl radicals or stable free radicals SET assays FRAP Ferric Reducing Antioxidant Power The reaction measures the reduction capacity of a ferric compound to a color end-product CUPRAC Copper Reduction Assay Variant of FRAP assay using Cu instead of Fe Folin-Ciocalteu assay Reduction of oxidized iron and molybdenum