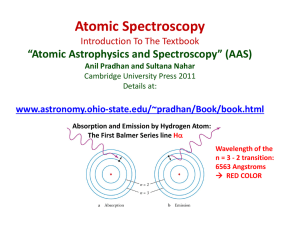
f (or n)
advertisement

Advanced Higher Chemistry Unit 1 - Electronic structure and the periodic table (20 hours) Unit 2 - Principles of chemical reactions (40 hours) Unit 3 - Organic chemistry (40 hours) Chemical investigation (20 hours) MM 2011 AH Electronic structure and the Periodic Table Electronic structure Electromagnetic spectrum & associated calculations Electronic configuration & the Periodic Table Spectroscopy Chemical bonding Covalent bonding Resonance structures Shapes of molecules & polyatomic ions Ionic lattices, superconductors and semiconductors Some chemistry of the Periodic Table The second & third short periods: oxides, chlorides & hydrides Electronic configuration & oxidation states of the transition metals Transition metal complexes MM 2011 AH Electromagnetic radiation James Maxwell’s theory • all radiation wave-like electric and magnetic fields in space Electromagnetic radiation • waves varying in length 10-14 m - 104 m • constant velocity in a vacuum 3 x 108 ms-1 MM 2011 AH Waves Waves can be described by their wavelength, frequency and velocity. Wavelength, l (m or nm; 1 nm = 10-9 m), the distance between two identical points on adjacent waves. Frequency, f (or) n (s-1 or Hz), the number of waves per second Velocity, c (ms-1 ), the distance a wave travels per second c = l x f (n) Wavenumber, 1/l (m -1 or cm one metre or one centimetre. -1 ), the number of waves in MM 2011 AH Waves continued l f (or n) = 3 Hz 1s f (or n) = 6 Hz l Velocity, c (ms-1 ) = l x f (or n) l=c f f (or n) = c l MM 2011 AH Waves summary Quantity Symbol Frequency Units Description Hz nm or m Wavelength Distance between adjacent waves Velocity Wavenumber c _ n l f (or n) Rate of advance of a wave Number of waves per second ms-1 cm-1 or m-1 m Number of waves in 1 m or 1 cm MM 2011 AH Waves summary Quantity Frequency Wavelength Velocity Wavenumber Symbol f (or n) Units Hz l m c _ n ms-1 cm-1 or m-1 Description Number of waves per second Distance between adjacent waves Rate of advance of a wave Number of waves in 1 m or 1 cm MM 2011 AH Electromagnetic spectrum radio & TV Wavelength, l/m 100 Wavelength, l/nm short microwaves 1 700 infrared rays 10-3 600 UV rays X-rays 10-6 10-9 visible gamma rays 10-12 500 400 Get X Unless Very Ill Men Start Resting Past papers 2013 Q2 -4; 2012 Q1 MM 2011 AH Quanta – small bundles or packets of energy. - fixed size so transfer of energy occurs in fixed amounts Electromagnetic radiation is also regarded as a stream of particles – photons (wave-particle duality). Energy of radiation for a photon can be calculated using E = h f or E = h c (E = h n) l h is Planck’s constant = 6.63 x 10-34 Js For one mole of photons E = L h f or E = L h c l (E = L h n) where L is Avogadro’s constant The energy of radiation is calculated in Jmol p4 LTS Q1 & 2 -1 or kJmol -1 MM 2011 AH