Electrochemistry - University of California, Santa Cruz
advertisement

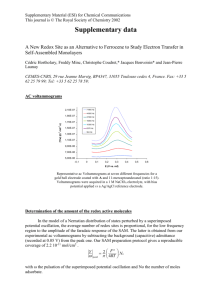
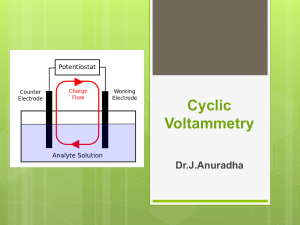
CHEM 146C_Experiment #8 Surface Electrochemistry: Adsorption of Polyoxometalate on Graphite Electrodes Yat Li Department of Chemistry & Biochemistry University of California, Santa Cruz Objective In this laboratory experiment, we will learn: 1. The basic concept of electrochemistry and cyclic voltammetry 2. How to study the electrochemical behavior of a surface-adsorbed redox species Electrochemistry Electrochemistry encompasses a group of qualitative and quantitative analytical methods based on the electrical properties of a solution of the analyte when it is made part of the electrochemical cell. • stiochiometry and rate of interfacial charge transfer • the rate of mass transfer • the extent of adsorption or chemisorptions • the rates and equilibrium constants for chemical reaction Electrochemical cell 1. Three electrode configuration • Working electrode: usually graphite; potential is varied linearly with time • Reference electrode: e.g. Ag/AgCl; potential remains constant throughout the experiment • Counter electrode: usually platinum coil, simply conducts electricity from the signal source through the solution to the working electrode 2. Supporting electrolyte: non-reactive electrolyte, conducts electricity 3. Analyte: e.g. redox species Cyclic voltammetry_excitation signal In voltammetry, a variable potential excitation signal is impressed on a working electrode in an electrochemical cell. Cyclic voltammetry: potential will be cycled between two potentials Same scan rate and region Triangular waveform Cyclic voltammograms For example, K3Fe(CN)6 A B: No current (no reducible or oxidizable species) B D: Fe(CN)63- + e- D F: Diffusion layer is extended away from electrode surface Fe(CN)64- F H/I: Reduction of Fe(CN)63- stop, current becomes zero again H/I J: Fe(CN)64- Fe(CN)63- + e- J K/A: Current decrease as the accumulated Fe(CN)64- used up Procedure_1 Record cyclic voltammograms of electrolyte solution with a clean graphite working electrode as a function of scan rate Procedure_2 Record cyclic voltammograms of electrolyte solution with a graphite working electrode modified with phosphomolybdic acid, as a function of scan rate Procedure_3 Record cyclic voltammograms of electrolyte solution with a graphite working electrode modified with phosphomolybdic acid as function of H2O2 concentration Cyclic voltammograms_quantitative information 1. Number of charge (Q) The integrated area under each wave represents the charge Q associated with the reduction or oxidation of the adsorbed layer Q=nFAΓ n: number of electrons F: Faraday constant A: the electrode surface area Γ: the surface coverage in moles of adsorbed molecules per surface area 2. Capacitance (C) The peak current is proportional to scan rate v, I = vC Icap: current v: scan rate Cd: capacitance Cyclic voltammograms_quantitative information 3. Number of electrons (n) For a reversible electrode reaction at 25 °C, the difference in peak potentials, DEp is expected to be DEp = │Epa - Epc│ = 90.6 / n 4. Surface coverage (Γ) When the number of electrons is known, the surface coverage can be calculated by the equation: Ipeak = n2F2vAΓ(4RT )-