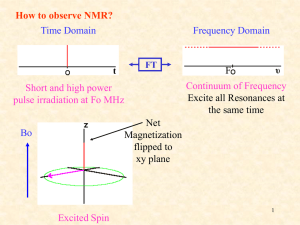
2D_NMR
advertisement

Highly Recommended NMR Book! Dr. Tim Claridge Director of NMR Spectroscopy & University Research Lecturer Organic Chemistry NMR Facility at the University of Oxford http://www.chem.ox.ac.uk/spectroscopy/nmr/ THE COSY (COrrelation SpectroscopY) EXPERIMENT Jean Jeener. 1971. Two-dimensional Fourier Transform NMR, presented at an Ampère International Summer School, Basko Polje, unpublished. A verbatim quote follows from Richard R. Ernst's Nobel Laureate Lecture delivered on December 2nd, 1992, ``A new approach to measure two-dimensional (2D) spectra has been proposed by Jean Jeener at an Ampere Summer School in Basko Polje, Yugoslavia, 1971 ([6]). He suggested a 2D Fourier transform experiment consisting of two 2 pulses with a variable time t1 between the pulses and the time variable t2 measuring the time elapsed after the second pulse as shown in Fig. 6 that expands the principles of Fig. 1. Measuring the response s(t1 t2) of the two-pulse sequence and Fourier-transformation with respect to both time variables produces a two-dimensional spectrum S(O1 O2) of the desired form (62,63). This two-pulse experiment by Jean Jeener is the forefather of a whole class of 2D experiments (8,63) that can also easily be expanded to multidimensional spectroscopy.'' THE BASIC IDEA OF nD NMR For a single uncoupled proton resonance P: Preparation E: Evolution M: Mixing D: Detection Amplitude modulation of a singlet resonance as a function of t 1 (signal decays due to spin relaxation) FT t2 FT t1 The concept can be expanded to 3 and n dimensions two-dimensional (2D) experiment threedimensional (3D) experiment In the previous slide two-dimensional experiment, a sample containing two uncoupled spins A and X, of offsets nA and nX, will produce 2D peaks at their corresponding chemical shift offsets in both dimensions (2D spectra are in fact 3D images). Contour Plot If spins A and X are J coupled, during the evolution time t1 the magnetization will evolve into anti-phase components. A necessary condition for the magnetization magnetization transfer to takes place during the mixing pulse (M). As a result, each magnetization (A and X) will not only modulate with its own resonance frequency but they will also modulate with the resonance frequency of the coupled spin, giving origin to a cross-correlation peak between them. Diagonal Peak Cross-Correlation Peak Jean Jeener’s brilliant idea has opened an endless avenue for what today is Multinuclear and Multidimensional NMR Spectroscopy. “Extensions of the standard COSY experiment. Relayed correlation, total correlation spectroscopy (TOCSY), and multiple quantum spectroscopy (MQS) increase the information content, while exclusive correlation (E.COSY), multiple quantum filtering (MQF), and spin topology filtration reduce the complexity. Both avenues can lead to three-dimensional spectroscopy.” Richard R. Ernst Nobel Lecture* *Richard R. Ernst. Nobel Lecture. http://nobelprize.org/nobel_prizes/chemistry/laureates/1991/ernst-lecture.pdf Angewandte Chemie Int. Ed. 1992, 31(7), 805-930. ADVANCE METHODS FOR STRUCTURE ELUCIDATION OF SMALL MOLECULES 2D NMR Spectroscopy We will focus mainly in 1H and 13C nuclei at natural abundance, although 2D NMR is not limited to these nuclei only. Correlations Through Chemical Bonds: -Homonuclear -Heteronuclear Correlations Through Space: -Homonuclear Through Chemical Bond 2D Homonuclear Chemical Shift Correlations Experiments COSY-90 Correlating coupled homonuclear spins. Typically used for correlating protons coupled over two or three bonds but may be used for any high-abundance nuclide. The basic COSY experiment. DQF-COSY Correlating coupled homonuclear spins. Typically used for correlating protons coupled over two or three bonds. Higher-resolution display than basic COSY. Additional information on magnitudes of coupling constants may be extracted from 2D peak fine structure. Singlets suppressed. COSY-b Correlating coupled homonuclear spins. Typically used for correlating protons coupled over two or three bonds but may be used for any high-abundance nuclide. Reduced 2D peak structure over basic COSY. Vicinal and geminal coupling relationships can be differentiated in some cases. Delayed COSY Correlating coupled homonuclear spins through small couplings. Often used to identify proton correlations over many bonds (>3), hence also known as long-range COSY. TOCSY Correlating coupled homonuclear spins and those that reside within the same spin system but which may not share mutual couplings. Employs the propagation of magnetization along a continuous chain of spins. Powerful technique for analyzing complex proton spectra. INADEQUATE Correlating coupled homonuclear spins of low natural abundance. Typically used for correlating adjacent carbon centers at natural abundance but has extremely low sensitivity. ADEQUATE Correlating coupled homonuclear spins of low natural abundance, primarily 13C–13C, but employing 1H excitation and detection for sensitivity improvement. The choice of the COSY Experiment. Which COSY should we use? Absolute-value (magnitude-mode) COSY-90 Pros: Simple and robust, magnitude processing well suited to automated operation Cons: Phase-twisted lineshapes produce poor resolution, which require strong resolution enhancement functions. Crosspeak fine structure not usually apparent Phase-sensitive COSY-90 Pros: High-resolution display due to absorptive lineshapes. Crosspeak fine structure apparent; J measurement possible Cons: Diagonal peaks have dispersive lineshapes that may interfere with neighboring crosspeaks. Requires high digital resolution to reveal multiplet structures Phase-sensitive DQF-COSY Pros: High-resolution display due to absorptive lineshapes. Crosspeak fine structure apparent; J measurement possible. Diagonals also have absorptive lineshapes. Singlets suppressed Cons:Theoretical sensitivity loss by a factor of 2 relative to the COSY-90 variant. Requires high digital resolution to reveal multiplet structure COSY-b Pros: Simple and robust. Magnitude processing well suited to automated operation. Simplification of crosspeak structures reduces peak overlap. Vicinal and geminal couplings can be distinguished in some cases from tilt of peaks Cons: Usually requires magnitude-mode presentation as phase sensitive variant has mixed-phase lineshapes Delayed COSY Pros: Enhances detection of small- and long-range couplings (< 2 Hz) such as between protons in allylic systems or those in w-relationships Cons: Requires magnitude-mode presentation. Crosspeaks due to larger couplings can be significantly attenuated Relayed COSY Pros: Provides two (or more)-step transfers and can reduce ambiguities arising from crosspeak overlap Cons: Typically has low sensitivity and responses show mixture of lineshapes, so magnitude-mode presentations may be required. TOCSY preferred TOCSY Pros: Provides multi-step (relayed) transfers to overcome ambiguities arising from crosspeak overlap. High sensitivity. In-phase lineshapes can provide correlations even in the presence of broad resonances Cons: Number of transfer steps associated with each crosspeak not known, a priori. In-phase lineshapes tend to mask crosspeak fine structure and may preclude J measurement COSY Magnitude Mode Gradient-selected COSY Best option No phase cycling One scan per increment is enough The Multiple Quantum Filter (MQF) AX Spin System E SQ ZQ Single Spin (Singlet) - 1/2 DQ b SQ SQ SQ SQ + 1/2 A1, A2, X1 and X2 are Single Quantum Transitions (SQ). Total Spin Change is 1 DQ: Double Quantum. Total Spin Change is 2 ZQ: Zero Quantum. Total Spin Change is 0 (zero) Phase Sensitive COSY *DQF: Double Quantum Filtered Phase Sensitive DQF-COSY* Phase Sensitive DQF-COSY Suppressed by DQF HDO MeOD Antiphase Absortive Structure 1D double-quantum filtration of the spectrum of the peptide Leu-enkephalin 5.4 in CD3OD. The singlet resonances of the solvent, truncated in the conventional 1D spectrum (a), have been filtered out in (b). The remaining peaks in (b) display the characteristic anti-phase multiplet structure (which may be masked by magnitude calculation if desired Multiplets Structure in Phase Sensitive DQF COSY AX Spin System AMX Spin System Overlapped resonances in the 1D Spectrum of the peptide Leu-enkephalin Resolved Multiplicity in the DQF COSY F2 slice. J extraction possible High Resolution DQF COSY COSY-b In the COSY-b experiment, the mixing pulse is usually set to 45o or 60o V: Vecinal J (Positive), G: Geminal J (Negative) Delayed COSY: detecting small couplings The delayed (or longrange) COSY sequence. Additional fixed delays are inserted into the basic COSY sequence to enhance the appearance of correlations from small couplings. The small J’s (1 Hz), COSY-90 Delayed COSY (D = 200ms) TOtal COrrelation SpectroscopY – TOCSY (Former HOHAHA) - Correlates all protons within the same spin system - Number of transfer steps associated with each crosspeak not known, a priori - A second significant feature of TOCSY that contrasts with COSY is that it utilizes the net transfer of in-phase magnetization; so it does not suffer from cancellation of anti-phase peaks under conditions of low digital resolution or large linewidths. In these instances, this feature makes TOCSY the more sensitive of these two methods. The TOCSY sequence. The spin-lock mixing time, m, replaces the single mixing pulse of the basic COSY experiment. VIRTUAL COUPLING Suppose that: - (Ha) is coupled to another (Hb) which is far away from it in chemical shift - Hence, we expect for Ha a simple doublet - But suppose that Hb is coupled to a third spin (Hc) which is very close to Hb in chemical shift. -Hence, we say that Hb and Hc are strongly coupled, which will distort the multiplet patterns of these two spins -The odd thing, however, is that the Ha multiplet becomes more complex, as if it were coupled to Hc as well as to Hb. This is called "virtual coupling" because the Ha nucleus, which has no J coupling to Hc, appears to be coupled to it because of the strong coupling between Hb and Hc A general way of stating this is that: Any nucleus which is coupled to one member of a strongly-coupled group of nuclei will behave as if it is J coupled to all of the members of the group” “ A classic example is the CH3 signal shape in fatty acids d 2.7 d 1.2 d 0.8 HO-C(O)-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH3 Broad distorted triplet VIRTUAL COUPLING CAN BE TEMPORARILY INDUCED BY SPIN-LOCK The spin-lock in its simplest form is a single, long, low-power pulse. This can be viewed as a continuous sequence of closely spaced 180 pulses bracketed by infinitely small periods, . Spin-Locked Magnetizations Chemical shift evolution terms are cancelled But not the J evolution terms Oscillating nature of the in-phase magnetization transfer in the TOCSY for protons Ha and Hb as a function of the mixing time t. t=0 Mxb Mx a t = 1/2J At 70ms full transfer for Jab= 7.14 Hz For Jab = 2 Hz, 18% of M transfer For Jab = 0.5 Hz, only 1.2% of M transfer Sample: Chentobiose Solvent: CDCl3 Spectrometer: AVANCE 400 TOCSY B A 1 Probehead: Inverse Broadband with z-Gradients 1 A Anomeric H B Anomeric H B 1H NMR A 1H NMR http://rmn.iqfr.csic.es/guide/tutorials/specdata/spectra/dis_tocsy.html Through Chemical Bond 2D Heteronuclear Chemical Shift Correlations Experiments HMQC (Heteronuclear Multiple Quantum Correlation) Correlating coupled heteronuclear spins across a single bond and hence identifying directly connected nuclei, most often 1H–13C. Employs detection of high-sensitivity nuclide, e.g. 1H, 19F, 31P (an ‘inverse technique’). Experimentally robust sequence, well suited to routine structural characterization. HSQC (Heteronuclear Single Quantum Correlation) Correlating coupled heteronuclear spins across a single bond and hence identifying directly connected nuclei. Employs detection of high-sensitivity nuclide, e.g. 1H, 19F, 31P (an ‘inverse technique’). Provides improved resolution over HMQC, so it is better suited for crowded spectra but can be more sensitive to experimental imperfections. HMBC (Heteronuclear Multiple Bonds Correlation) Correlating coupled spins across multiple bonds. Employs detection of high-sensitivity nuclide, e.g. 1H, 19F, 31P (an ‘inverse technique’). Essentially HMQC tuned for the detection of small couplings. Most valuable in correlating 1H–13C over two or three bonds. Powerful tool for linking together structural fragments. HETCOR (Heteronuclear Correlation) Correlating coupled heteronuclear spins across a single bond. Employs detection of the lower- nuclide, typically 13C, so has significantly lower sensitivity than inverse techniques. Benefits from high resolution in 13C dimension, so may find use when this is critical, otherwise superseded by above methods. H–X–Y Triple-resonance methods for correlating protons and a heteratom (Y) whilst using a second heteroatom (X) to either relay the correlations or edit the correlation spectrum. 13C (CARBON) NMR SPECTROSCOPY Some facts: - NMR is not limited to study protons, you can also observe 13C, 31P, 15N, Si29, etc. - 13C has nuclear spin I=1/2 (same as proton) - 13C natural abundance is 1.109%. Hence, the probability of having a 13C in a molecule is 1 (one) 13C in 100 (hundred) 12C. -The probability of having 2 (two) carbons in a molecule is 0.01 x 0.01 = 0.0001. So 1 in 10000 12C!!!... That is why you do not see 13C coupled to other 13C atoms. A fact that makes the 13C NMR spectrum much more simpler. - Just imagine a proton NMR spectrum if the natural abundance of 13C were 100%. - The 1H g/2p is 42.5781 MHz/Tesla and the 13C g/2p is 10.71 MHz/Tesla - Their relative individual receptivity at same abundance is: 1H g3 / 13C g3 = (42.5781)3/(10.71)3 = 62.83 At natural abundance is 62.83 / 0.01109 = 5666 THE POWER OF CHEMICAL SHIFT DISPERSION OF 13C NMR (AS WELL AS OTHER X NUCLEI) 1H NMR Spectrum of Cholesterol (300 MHz – CDCl3 – 300K) C27H46O H-6 5.5 5.0 H-3 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 1H NMR Spectrum of Cholesterol (300 MHz – CDCl3 – 300K) All we can identify at first glance is: 5 CH3 and 2 CH groups. Only 17 out of 46 total protons in the molecule 1.05 1.00 0.95 0.90 0.85 0.80 0.75 0.70 ppm 13C NMR Spectrum of Cholesterol (75 MHz – CDCl3 – 300K) CDCl3 C-O 3 2 1 C=C 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm 13C NMR Spectrum of Cholesterol (75 MHz – CDCl3 – 300K) 10 6 4 5 7,8 11 13 9 14 12 42.4 59 58 57 56 55 54 53 52 51 50 49 48 42.3 47 46 ppm 45 44 43 42 41 40 39 38 37 ppm 13C 15,16 NMR Spectrum of Cholesterol (75 MHz – CDCl3 – 300K) We can count all but two overlapped signals. Note that the two overlapped signals have twice the height of the rest. 18 17 32 31 30 29 22 20 21 23 24 19 28 27 26 25 24 23 22 21 26 27 25 20 19 18 17 16 15 14 13 ppm DMSO-d6 1D 13C NMR, DMSO-d6, 125MHz Soluble part of 1mg of dye 10 (lots of sample precipitate) 36 hours S O + N N O O dye 10 190 180 170 S 160 150 O 140 x64 x64 - 130 120 110 100 90 80 70 60 50 40 30 20 ppm Dye 10 gp-COSY-90 Magnitude 500 MHz 2 scan per F1 increment 512 increments 20 minutes Edited HSQC (1H:500MHz, 13C:125MHz) 16 scans per F1 increment 256 increments in F1 (Echo-Antiecho) 90 minutes SENSITIVITY IN HETERONUCLEAR 2D NMR SPECTROSCOPY -The major concern for chemist is the horrible low sensitivity of NMR compared to other spectroscopies For experiments involving spin ½ nuclei: N: number of molecules in the observed sample volume A: a term that represents the abundance of the NMR-active spins involved in the experiment, T: temperature B0: the static magnetic field, gexc and gobs represent the magnetogyric ratios of the initially excited and observed spins, respectively T2*: the effective transverse relaxation time S: is the total number of accumulated scans Relative Sensitivity of Different 2D Schemes P: preparation E: evolution M: mixing D: detection 1D Carbon/DEPT NMR or HSQC/HMQC/HMBC? Directly observing carbon (13C) at natural abundance does not longer make any sense if they can be observed through the much more sensitive 1H nuclide using inverse detection NMR probes. Just imagine experiments that can combine the sensitivity of 1H with the chemical shift dispersion of 13C. Those experiments are basically the HMQC (Heteronuclear Multiple Quantum Correlation), HSQC (Heteronuclear Single Quantum Correlation) and the HMBC (Heteronuclear Multiple Bond Correlations) ISOTOPOMERS AND ISOTOPOLOGUES According to IUPAC: Isotopomer Isomers having the same number of each isotopic atom but differing in their positions. The term is a contraction of 'isotopic isomer'. Isotopomers can be either constitutional isomers (e.g. CH2DCH=O and CH3CD=O) or isotopic stereoisomers [e.g. (R)- and (S)-CH3CHDOH or (Z)- and (E)-CH3CH=CHD]. Isotopologue A molecular entity that differs only in isotopic composition (number of isotopic substitutions), e.g. CH4, CH3D, CH2D2. IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook. 1 1 12 12 H H Cl C C Br 1H-NMR Cl Br A 1 1 13 12 98% C C 1 12 13 H H H H Cl 1 Br 13C NMR HSQC/HMQC/HMBC Cl C C Cl Br Cl Br B 1% C 1% 1 H 1H Cl 13 C 13 C Cl Br D 0.01% Br INADEQUATE Br HMQC or HSQC? Lets See Unwanted 1H-1H Evolutions in F1 Better Resolution in F1 At this point there are no questions that HSQC is the best option over HMQC!!! Now, the next question is: What HSQC experiment should you use? 1) Use a gradient version of the experiment. Gradients can be used to destroy magnetization that would otherwise lead to artifacts. Hence you will obtain cleaner spectra. 2) Use a version that includes sensitivity enhancement in the pulse program. Sensitivity matter, particularly when you have limited amount of sample, or just want to do it faster. 3) Use the edited version of HSQC. By the same token you will obtain carbon multiplicity information (CH, CH2, CH3). 4) Finally, use a version that includes adiabatic pulses for 13C inversion and refocusing. You will remove those very nasty phase artifacts at the resonance edges of the carbon chemical shifts. Versions that accounts for all these points are now standard and are available in the Bruker pulse program library. Setting up this experiments is no longer problematic. They are routinely run in my laboratory. Comparison of 12C-1H signal suppression methods used in proton detected heteronuclear correlation experiments Clean 12C-1H (a) Conventional 1D proton spectrum without suppression of the parent resonance and displays the required 13C satellites. (b) Phase cycling (c) Optimised BIRD presaturation (d) Pulsed field gradients to remove the parent line (Notice loss of sensitivity) Echo-Antiecho Gradient HSQC Echo-Antiecho Gradient HSQC with Sensitivity Enhancement (PEP) x2 Gain in Senstitivity Editing based on multiplicity DEPT-HMQC D = 1/2J Edited HSQC Edited HSQC CH2 (Black) CH and CH3 (Red) Simulation with NMRSim At 125 MHz in 13C, 200 ppm corresponds to 25kHz HSQC: Sensitivity Enhancement with Square 13C 180 degree pulses HSQC: Sensitivity Enhancement with Adiabatic 13C 180 degree pulses Adiabatic Inversion Adiabatic Refocusing Hymenistatin Heteronuclear Multiple-Bond Correlation Spectroscopy (HMBC) HMBC is in principle an HMQC experiment tuned for small 1H-13C coupling constants 12a Magnitude Mode HMBC with low-pass filter 12b 14 C7 2 3 4 10 9 1 8 5 6 O H One-Bond Cross-Peak O 15 7 11 13 O 1 C11 Ludartin C13 12 Through Space 2D Homonuclear Chemical Shift Correlations Experiments NOE difference Establishing NOEs and hence spatial proximity between protons. Suitable only for ‘small’ molecules (Mr << 1000), for which NOEs are positive. Observes steady-state or equilibrium NOEs generated from the saturation of a target. NOESY(2D or 1D) Establishing NOEs and hence spatial proximity between protons. Suitable for ‘small’ (Mr << 1000) and large molecules (Mr > 2000) for which NOEs are positive and negative respectively, but may fail for mid-sized molecules (zero NOE). Observes transient NOEs generated from the inversion of a target. Estimates of internuclear separations can be obtained in favourable cases. ROESY(2D or 1D) Establishing NOEs and hence spatial proximity between protons. Suitable for any molecule but often essential for mid-sized molecules; NOEs are positive for all molecular sizes. Observes transient NOEs in the rotating-frame, but is prone to interference from other mechanisms so requires cautious interpretation. Estimates of internuclear separations can be obtained in favourable cases. HOESY Establishing heteronuclear NOEs and hence spatial proximity between different nuclides, for example, 1H–13C. Can provide useful stereochemical information when homonuclear NOEs are insufficient or inappropriate. Suffers from low sensitivity but 1H detected variants can help. EXSY (2D) Qualitative mapping of exchange pathways in dynamic systems when exchange rates are slow on the NMR chemical shift timescale, meaning separate resonances are observed for each exchanging species. Quantitative data on exchange kinetics can be obtained in favorable cases. NOESY OR ROESY?