Hydrophobic interaction chromatography
advertisement

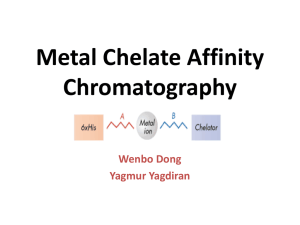
Hydrophobic interaction chromatography Umair Saleem Methods in protein chemistry ■ Alternatives • Gel filtration chromatography • Ion exchange chromatography • Reverse phase chromatography Why HIC? • Different basis of separation • Weaker interactions → Less structural damage → Maintain high activity Contents → Purpose → Principle of HIC → Advantages of using HIC → What are the factors affecting HIC → Conclusion Source of protein Extraction Separation Purity & characterization ■ Purpose • Downstream purification • Separation of biomolecuoles • Exploits differences in hydrophobicity. → Number of hydrophobic aminoacids. → Distribution of these aminoacids. Principle • • Separation of substances is based on their varying strength of interaction with hydrophobic groups attached to an uncharged gel matrix Hydrophobic groups on proteins are sufficiently exposed to bind to the hydrophobic groups on the matrix. • How is this achieved? Source of protein Extraction Separation Purity & characterization General Concept Hydrophobic Interaction Chromatography Experimental Technique ■ Choice of column → XK colums for HIC. • Column dimensions → Short bed height (5-15 cm) suitable for HIC ■ Packing of Column: a modern, highly crosslinked agarose-based gel such as Sepharose Fast Flow is however easier than packing a gel filtration column since the bed height required is much smaller. ■ Sample preparation • Sample composition • Sample volume • Sample viscosity ■ Sample application ■ Batch Separation Advantages of HIC Large volume of sample can be loaded Samples with high ionic strength can be used Well suited to use before gel filtration, ion-exchange and affinity chromatography Sample eluted with low salt Purification steps that generate large sample volume can be coupled with this method Good for samples after ammonium sulfate fractionation. These techniques may require pretreatment of samples (e.g. reducing ionic strength) Sample can be used in ion exchange chromatography step Factors affecting HIC Type and concentration of ligand Type of base matrix. Type and concentration of salt pH Temperature Additives HIC Ligands OH O CH 2 CH CH 2 O ( CH 2) 3 CH 3 Butyl OH O CH 2 CH CH 2 O ( CH 2) 7 CH 3 Octyl OH O CH 2 CH CH 2 O Phenyl Effects of ligand density Degree of substitution • ‰ Binding capacity of protein to HIC increases with increased alkyl chain length (A) and increased degree of substitution of immobilized ligand (B) • ‰ Caution: protein can bind via multipoint attachment, thus difficult to elute Type of base matrix Important to take note that selectivity will not be exactly the ‰ same even with the same type of ligand if the base matrix is different Two widely used supports are cross-linked agarose and ‰ synthetic copolymer materials May be necessary to modify adsorption and elution conditions ‰ Influence of salts Type of salt salt effect follow the Hofmeister series. Hydrophobic interaction increases at increased salt concentration Increasing salting out effect Anions: PO43- >SO42- >Cl- >Br- >NO3- >ClO4>I- >SCNCations: NH4+ > K+ >Na+ >Li+ >Mg2+ Decreasing surface tension Increasing chaotropic effect PH Effect of pH on HIC is not straight forward. I‰ n general an increase in pH weakens hydrophobic interactions. It could be due to increased titration of charged groups leading to an increase in hydrophilicity of the proteins Decrease in pH leads to an apparently increase in ‰ hydrophobic interaction I‰ mplication: Important factor to consider for optimization of HIC interaction. It is observed that proteins which do not bind to HIC adsorbent at neutral pH, bind at acidic pH. Temperature Visser and Strating (1975): that role of temperature is a complex issue and differ from observation of Hierten. ‰ Binding of proteins to HIC adsorbents is entropy driven (Hjerten, 1976), i.e. interaction increases with increase in temperature ‰ Discrepancy in views could be due to differential effects by temperature on the conformational state of different proteins and solubility in solution ‰ Practical terms: To be aware that procedure developed at room temperature may be different if used in the cold room Additives Salts that cause “salting-in” will weaken protein-ligand interactions. A ‰ lcohols and detergents (non-polar parts) can compete with protein for HIC absorbent sites and may displace proteins. Hydrophobic Interaction Chromatography Overall Conclusions Very useful technique for mAb purification Mainly used in the third step as a complementary technique to protein A and IEC (in-vivo) HIC can be used in both binding and removal mode Can be a useful alternative to SEC for aggregate removal HIC is also very useful for purification of antibodies in 2-step techniques (non-protein A) for in-vitro applications. THANKS