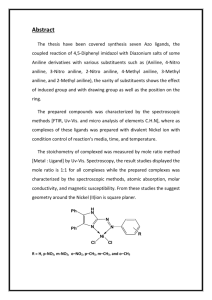
Acids of Bases
advertisement

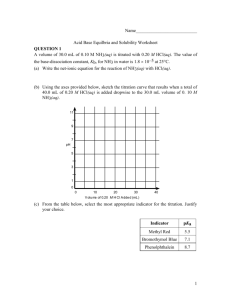
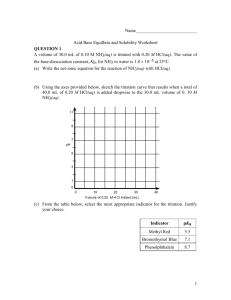
Acids and Bases 2003 AP #1 A-E Write the Equilibrium, Kb, for the reaction represented Writing an equilibrium expression for 1a Using the law of mass action given the chemical equilibrium equation Concentration Products over Reactants raised to their stoichiometric coefficients excluding pure liquids and solids! Writing an equilibrium expression for 1a In this case, equilibrium expression consists of the products of the concentrations of Conjugate acid of Aniline and Hydroxide Ion over the concentration of Aniline. A Sample of aniline is dissolved in water to produce 25mL of a 0.10M. The pH of the solution is 8.82. Calculate the Equilibrium Constant Calculate the Kb for the reaction Use the equilibrium expression above to determine the Kb. The concentration of aniline is 0.1M The Hydroxide and Conjugate acid concentration can both be determined after calculation of the pOH (14-8.82). Take the pOH and raise it to the 10^(-pOH) Calculate the Kb for the reaction This will yield the concentration for OHand Aniline Conjugate because they are produce in the same proportion 1:1. Multiply these concentrations and divide them by the initial concentration of Aniline. Calculate Kb Assume the initial concentration of [OH-] is negligible. The reason x is not subtracted from the initial concentration of aniline is because x is so small that it is considered negligible. The solution prepared in part b is titrated with a 0.10M. Calculate the pH of the solution when 5.0mL of the acid has been added. Calculate pH after 5mL of HCl is added Set up a net chemical equation for the reaction of Aniline and H+ (Strong Acid) Since the initial concentrations of OH- and conjugate acid are small they are not taken into account. Multiply the Molarity of Aniline by the volume in liters (same with the HCl) From here you have the moles of both Aniline and HCl Calculate pH after 5mL of HCl is added The H+ will combine with Aniline to form a conjugate acid and goes to completion. Subtract the number of moles of H+ from the moles of Aniline. This gives a new number of moles of Aniline. Since all of the H+ moles are consumed, it is equal to the number of moles of the Conjugate acid. Calculate pH after 5mL of HCl is added To determine the pH, we will use a variation of the Henderson-Hassalbauch. Below From here we take the pKb calculated above and the mole ratio of the acid over the base. The pOH can be determined To get the pOH we subtract the pOH from 14 to get the pH. Calculate the pH at the equivalence point. pH at the equivalence point We already know the moles of the Aniline. The moles of Aniline must equal the moles of HCl for the equivalence point to be reached. (Ratio 1:1) From here we have the only Aniline Conjugate Acid moles. We must determine the concentration of conjugate acid and the Ka of the conjugate pH at the equivalence point 25mL initially of aniline To determine the volume of HCl take the moles of HCl and divide it by the concentration which yields the volume required. Convert all volumes to liters and add the initial volume of aniline with the volume of HCl added. Take the moles of conjugate acid and divide it by this new volume pH at the equivalence point To determine the Ka dived the Kb into (1.0 x 10^-14). pH at the equivalence point Write the Chemical reaction for the behavior of Aniline conjugate in water and make an equilibrium expression. x is not subtracted from the initial concentration because it is considered negligible. Multiply the concentration of Conjugate aniline concentration by the Ka. Then take the square root of the product. pH at the equivalence point Then take –log (x) of the answer This will give you the pH at the equivalence point pH = 2.97 Which of the following indicators listed is most suitable for this titration. Selecting an Indicator Based on the calculations above the pH at the end point is 2.97 Erythrosine is optimal because based on the color change in an acidic pH It is a weak based titration with a strong acid. 2002 Calculate the value [H+] in an HOBr solution that has a pH of 4.95 Calculate [H+] Given the pH is 4.95 Use the exponent 10 raised the –pH This will yield the [H+] Write the equilibrium constant expression for the ionization of HOBr in an HOBr solution with a [H+]= 1.8 x 10^(-5) Equilibrium constant expression Given the chemical equilibrium reaction and the concentration of [H+] [H+] = [OBr-]: This is true because as HOBr dissociates the products form in same proportions Exclude x because it is negligible. Use the Law of Mass action Products over Reactants raised to their stoichiometric coefficients. Use the equilibrium constant. The product of the concentration divided by the Ka yields the concentration of HOBr. Calculate the volume of 0.115M Ba(OH)2 needed to reach equivalence when titrated into 65mL sample of 0.146M of HOBr Calculate Volume Needed to Reach Equivalent Point Convert the Volume of HOBr to liters Multiply the volume in Liters by the Molarity of HOBr in Solution This will give you the moles of the HOBr in solution The titrant used is Ba(OH)2 (Strong Base) so the concentration of OH- must be doubled therefore you must multiply the concentration of Ba(OH)2 to get the concentration of OH- Calculate Volume Needed to Reach Equivalent Point Take the Moles of HOBr (HOBr = OH-) that was calculated and divide it by the molarity of OH([Ba(OH)2] x 2). This will give you the volume in liters necessary to added to reach eq point Indicate whether the pH at equivalence point is less than 7, 7, or greater than seven. Explain What will the pH at the eq point be? You can go through the calculations to determine the pH specifically at the eq point. The basic rule of thumb is that if you are titrating a weak acid with a strong base then the pH at the end point will be greater than 7. Calculate the number of the moles of NaOBr(s) that would have to be added to 125mL of 0.160M HOBr to produce a buffer solution with a [H+] of 5.00 x 10^(-9) Number of Moles needed to produce certain Concentration The HendersonHasselbauch equation can be used for this scenario (See equation Below) Given the [H+] take the –log([H+]) and determine the pH for the equation Number of Moles needed to produce certain Concentration You have already been given the Ka so plug that in the equation as well Separate the ratio of concentrations into log(B) – log (A) (Refer to Log Rules) Number of Moles needed to produce certain Concentration Solve for the Log (Base) and then eliminate the log function by raising the solution using the base of 10. This will yield your concentration of Base Number of Moles needed to produce certain Concentration Multiply the concentration by the volume of solution in liters This will yield the number of NaOBr moles HOBr is a weaker acid than HBrO3. Account for this fact. Strong Acid/Weak Acid The number of oxygen atoms will effect the strength of the acid. HOBr has a single oxygen atom while HBrO3 contains three. Charge of X Oxygen is a very electro negative atom and will draw electrons away from the H-Br bond thus weakening the bond making it easier to dissociate when in dissolved in water. The more oxygen atoms the weaker the H-Br bond. The charge on the X which in this case is the Br atom has a different charge in HOBr (+1) than in HBrO3 (+5) Strength of O-H Bond Strength of X-O Bond Arrhenius Acid Base Concept Arrhenius Acid/Base Concept Acids produce Hydrogen ions (H+) within an aqueous solution Bases produce Hydroxide Ions (OH-) in solution Definition is limited because it applies only to acids and bases that can dissociate OH- and H+ ions Examples: - NaOH will dissociate into Na+ and OHHCl will dissociate into H+ and Cl- Bronsted-Lowry Model The model definition of Acid/Base Bronsted Acid – A proton “donor” Bronsted Base – A proton “acceptor” The definition applies to many more molecules that may exhibit Acid/Base qualities but do not directly produce OHor H+ ions. Every Acid and Base has a conjugate Acid or Base Water can act as an acid and a base H3O+ (Hydronium ion) (acid) and OH- (Base) Bronsted-Lowry Model Example of Bronsted Base NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) Base Acid Conjugate Acid Conjugate Base Example of Bronsted Acid HC2H3O2(aq) + H2O(l) H3O+(aq) + C2H3O2-(aq) Weak Acid Base Conjugate Acid Conjugate Base Lewis Acid/Base Definition Lewis Acid/Base Definition Lewis Acid – Electron Pair Acceptor Lewis Base – Electron Pair Donor Encompasses an even wider variety of molecules (Bronsted and Arrhenius) even ones that do not donate protons or produce OH- ions. Must be aware of the Lewis structure of a particular molecule to determine whether it is a Lewis Acid or Base. Lewis Acid/Base Definition Examples BF3(g) + NH3(g) F3BNH3(g) BF3 is the Lewis Acid because it has no free unpaired electrons with only has 6 electrons around the central atom (Boron will require one more pair of electrons to complete the valence shell) NH3 is the Lewis Base because the molecule has a completed octet valence shell with free unpaired electrons on the central atom. The BF3 will accept these unpaired electrons and form a covalent bond. Lewis Acid/Base Definition Example Ni2+(aq) + 6NH3(aq) [Ni(NH3)6]2+(aq) Ni2+ is the Lewis Acid because it is a cation which will attract negatively charged electrons to toward itself. The NH3 is the Lewis Base because it provides the free unpaired electrons for the Ni2+ Lewis Acid/Base Definition Example Even earlier definitions are encompassed in the Lewis Acid Model H+ + H2O H3O+