Pankaj B. Desai, PhD
advertisement

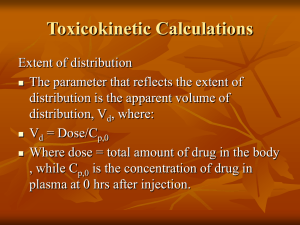
Key Pharmacokinetic Concepts – Single Dose and Steady State Drug Administration Pankaj B. Desai. Ph.D. Professor of Pharmacokinetics and Biopharmaceutics Director, Drug Development Graduate Program Morning Agenda: Wake Up and Smell the Coffee (Cytochrome P450 1A2 Substrate) CYP1A2 Substrate Overview of ADME principles Important PK Parameters First Pass Metabolism Compartmental & NonCompartmental Analyses Single Dose Kinetics Multiple Dose Kinetics Drug-Drug Interactions Inter-Subject Variability ADME ADME ISSUES IN ANTIssues I-CANCER DRUG DEVELOPMENT ADME Clinical Pharmacology • First in Human -Pharmacokinetically Guided Dose Escalation/ Drug Tolerance Study • Pharmacokinetics-Pharmacodynamics • Drug Metabolism • Mass Balance with Radiolabeled Compounds • Bioequivalence:Generic compounds • Single and multiple doses • Conventional versus controlled release formulations • Bioavailability of metabolites • Drug-Drug/Drug Dietary Product Interactions • Special Populations Drug Input & Different Routes of Administration 1. 2. I.V. and I.A. injections: • Bolus dosing • Zero-Order Input (Infusions) Extravascular Administration • First Order (mostly passive diffusion) • Zero Order (active transport and controlled release systems) Factors Affecting Drug Distribution • Phyisco-chemical properties of the drug • Small vs. Large mol.wt. Compounds • Hydrophilic vs. Lipophilic compounds • pH of the milieu and pKa of the drug • Perfusion rate (blood flow/min/g tissue) • Protein binding • Anatomical restrictions • • • • CNS- protected by the blood brain barrier Transport across placenta Salivary Drug Excretion (S/P ratios) Excretion of the drug in milk (M/P ratios) Apparent Volume of Distribution • Mathematical term to correlate amount & concentration • Merely a tool to understand the EXTENT of drug distribution- not a real physiological volume • Compare to the volume of body waters • Best calculated from I.V. Dosing as I.V. Dose/Cpo Drug Sulfisoxazole Phenytoin Phenobarbital Diazepam Digoxin L/Kg 0.16 0.63 0.55 2.4 7 L/70 kg 11.2 44.1 38.5 168 490 Apparent Volume of Distribution Plasma Water-3.5 L, ~4.5 % body wt (w/w) 100 mg Conc = 2 mg/ml Vd = 50 ml Beaker without Charcoal 100 mg Conc = 0.2 mg/ml Vd = 500 ml Beaker with Charcoal T B W E C W Total extracellular water - 15 L, 20 % body wt (w/w) Total Intracellular water –20 L, 30 % body wt (w/w) Total body Water 40 L, ~55 % body wt (w/w) Major Drug Elimination Pathways (Coordinated defense mechanism) Biotransformation HEPATIC Excretion Extra-Hepatic Renal Phase I Phase II Biliary Glomerular Filtration • Kidney receives 1.1 L of blood (20 – 25%) of cardiac output • 10 % is filtered at the glomerulus • Compounds with Mol.wt < 20,000 filtered • GFR = 120 ml/min • CLR of Inulin - a measure of GFR • Filtered freely into the tubule • Not influenced by protein binding and neither secreted nor reabsorbed • Rate of filtration = Fu. Cp.GFR • Not a very effective drug extraction process (maximal ~ 0.11 or 10 %) Active Secretion • • • • Detected when the overall rate of urinary drug excretion exceeds the rate of filtration Secretory processes (proteins) located predominantly within the proximal tubules Mechanisms exist for secreting acids (anions) and bases (cations) from plasma into the tubular lumen • Energy-dependent • Saturable processes • Subject to competitive inhibition Effect of Protein-Binding • Depends upon secretion efficiency and contact time at the secretory sites • Restrictive (dependent on the Fub) vs. Non-Restrictive (perfusion-rate limited) Reabsorption • Must occur when CLR < fu.GFR • Reabsorption occurs all long the nephron, associated with reabsorption of water; majority however occurring from the proximal tubules • Predominantly a passive diffusion process • Driven by concentration-gradient across the tubular lumen • Active secretion occurs for many endogenous compounds such as vitamins, electrolytes, glucose and amino acids • Urine-Plasma Ratio (U/P) based on HendersonHasselbalch equation • Influence of pKa and pH of urine Major Tissues Involved in Drug Metabolism • • • • • Liver Small intestines Kidney Lung Other portals of entry into the body and protected organs. -e.g. nasal mucosa Representation of drug metabolism and excretion by the hepatocyte Biliary Excretion is Transporter Mediated Phase I and Phase II Drug Metabolizing Enzymes Phase I enzymes: Predominantly cytochrome P450 (CYP) Drug Metabolism by CYPs Theophylline, caffeine, Olanzapine CYP2C8 Paclitaxel Rosiglitazon e CYP2C9 cerivastatin(15%) Includes: warfarin phenytoin tolbutamide Losartan CYP2A6 (Coumarin) CYP2E1 (Chlorzoxazone) CYP2B6 CYP1A2 bupropion, 5% tamoxifen, efavirenz CYP3A (50%) Includes: lovastatin cyclosporin nifedipine midazolam CYP2D6 ethinylestradiol (25%) Ritonavir Includes: Tricyclic antidepressants, Midazolam SSRI's, haliperidol, propanolol, atomoxetinetestosterone Detxromethorphan, Phase II Reactions • Also known as Synthetic (conjugation) reactions • Major reaction: Transfer of the conjugating moiety to the drug • Enzymes involved are “transferase” • • • • • • Glucuronosyl transferase Sulfotransferases N-acetyltransferase Methyltransferase Glycine transferase Glutathione-S-transferase Drug Biotransformation Reactions • Active Drug to Inactive Metabolite • Amphetamine • Phenobarbital • Taxol Phenylacetone Hydroxyphenobarbital 6-hydroxytaxol • Active Drug to Active Metabolite • Codeine • Procainamide • tamoxifen Morphine N-acetylprocainamide 4-hydroxytamoxifen Drug Biotransformation Reactions • Inactive Drug to Active Metabolite • Hetacillin • Sulfasalazine • Cyclophosphamide Ampicillin Sulfapyridine + 5 ASA Nitrogen mustard • Active Drug to Reactive Intermediates • Acetaminophen Reactive metabolites (hepatic necrosis) • Benzo(a)pyrene Reactive metabolite (carcinogenic) Nomenclature • Basis: Amino acid sequence • Families: Less than 40 % a.a. sequence assigned to different gene families (gene families 1, 2, 3, 4 etc.) • Subfamilies: 40 – 55 % identical sequence (2A, 2B, 2C, 3A etc.) CYP3A4 Family Subfamily Isoform CYP Nomenclature (Contd.) • • • • • • Cytochrome P450 Nomenclature, e.g. for CYP2D6 CYP = cytochrome P450 2 = genetic family D = genetic sub-family 6 = specific gene NOTE that this nomenclature is genetically based: it has NO functional implication Examples of CYP mediated Oxidative Examples of reactions catalyzed by cytochrome P450: Metabolism Hydroxylation of aliphatic carbon Examples of CYP mediated Oxidative Heteroatom dealkylation Metabolism Examples of reactions catalyzed by cytochrome P450: Clearance Concepts Compartmental Modeling One-Compartment Open Model I.V. bolus DB1 Cp1 Vd k10 K10 = overall Elimination Rate Constant I.V. Bolus k 10 t Cp C p e D Cp Vd Two-compartment Open model λ t λZt Cp C1 Cz 1 Central or Plasma I.V. bolus Cp1 VC Dp k12 k21 Tissue Dt Ct Vt 1- hybrid rate constant (distribution) z- hybrid rate constant (terminal) Two-compartment Open Model Elimination only Blood flow to human tissues Tissue Percent Body Weight Percent Cardiac Output Adrenals 0.02 1 550 Kidney 0.4 24 450 Liver 2.0 25 Hepatic Portal Blood Flow (ml/100 g tissue/min) 5 20 20 75 Brain 2.0 15 55 Skin 7.0 5 5 Muscle (basal) 40.0 15 3 Connective Tissue 7.0 1 1 Fat 15.0 2 1 Extravascular dose e.v. dose Dp Cp Vd ka Site of absorption k10 16 Cp' Conc(ug/ml) 12 8 Cp 4 Cp'-Cp 0 0 5 10 Time(hrs) Cp= F.Dose.Ka V(ka-k) (e-k.t- e-ka.t) 15 NCA Used to estimate • AUC • Bioavailability • Clearance • Volume of Distribution • Average Steady State Concentration AUC Trapezoidal Rule AUC= ½(t3-t2)(C2+C3) AUC Example AUC(0-) AUC(ug.hr/ml) 10 0 0.25 0.69 2.40 7.41 18.11 17.46 14.51 11.41 8.71 6.46 19.38 106.80 8 Conc(ug/ml) Conc (ug/ml) 0 2.025 3.53 6.07 8.75 9.36 8.1 6.41 5 3.71 2.75 6 4 2 0 0 2 4 6 8 10 12 14 16 Tim e(hr) Cp(last)= 2.75/0.1419 Conc Time Profile (Oral Dose) y = 20.245e-0.1419x 10 R2 = 0.9981 Conc (ug/ml) Time (hr) 0 0.25 0.5 1 2 4 6 8 10 12 14 1 0 2 4 6 8 Time (hr) 10 12 14 16 Bioavailability • Absolute Bioavailability • Relative Bioavailability F= [AUC]e.v/[DOSE]e.v [AUC]i.v/[DOSE]i.v F= [AUC]e.v/[DOSE]e.v [AUC]std/[DOSE]std Bioequivalence • Two products are considered to be bioequivalent if the concentration time profiles are so similar that they are likely to produce clinically relevant differences in either efficacy or toxicity. • Common measures used to assess differences are Tmax, Cmax and AUC. Other Parameters • CL = Di.v/AUC • AUMC = ½(t2-t1)(C1t1 +C2t2) • MRT (Mean Residence Time) = AUMC/AUC or MRT = 1/K or CL/V • Vss = CL. MRT Multiple Dosing –Overall Aims • Key Concepts • Principle of Superposition • Drug Accumulation and Steady State • Persistence Factor and Accumulation Factor • Peak, Trough and Steady State Average Levels • Applications • Determination of drug concentrations and amounts following multiple i.v. and e.v. doses (Ka > > K10) » max, min and during a dosing interval • Determination of dosing regimens – Doses (Maintenance and Loading) and Dosing Interval » Cpmax consideration » Cpmin consideration » Cpmax and Cpmin consideration • Practical Considerations in Decision Making Drug Accumulation Depends on Frequency of Administration Multiple I.V. Dosing The AUC within a dosing interval at steady state is equal to the total AUC of a single dose. Peak, Trough and Css Average Accumulation Index - Cssmax/Cmax1 AUC at Steady State = AUC0 ∞ Impact of Half-life and dosing interval Half-Li on Goals of the Dosing Regimen Dosing Regimen: Loading and Maintenance Doses Constant Rate Regimens Sources Sourcesof of Variability Variability • • • • • Genetic factors • Genetic differences within population • Racial differences among different populations Environmental factors and drug interactions • Enzyme induction • Enzyme inhibition Physiologic considerations • Age • Gender • Diet/nutrition • Pathophysiology Drug dosage regimen • Route of drug administration Dose dependent (nonlinear) pharmacokinetics Examples of CYP3A Inducers Therapeutic Class Anti-epileptic Drugs Anti-Infective Agents Anti-Cancer Drugs Carbamazepine Phenobarbital Phenytoin Topiramate Felbamate Rifampicin Paclitaxel Rifabutin Docetaxel Rifapentine Cyclophosphamide Clotrimazole Ifophosphamide Sulfadimidine Tamoxifen Suflinpyrazone 4-hydroxyEfavirenz tamoxifen Amprenavir SU5416 Nelfinavir Ritonavir Capravirine Miscellaneous Lovastatin Troglitazone Omeprazole Prednisolone Probencid Phenylbutazone Diazepam fexofenadine Hyperforin Induction of CYP1A2 (Ethoxyresorufin O-deethylase) by SU5416 in Primary Human Hepatocytes Stopeck et.al. Clin. Cancer Research, 2002 Salzberg et.al, Investigational New Drugs 24: 299–304, 2006) Example of Auto-Induction – SU5416 Oral Treatment AUC Day 8 AUC Day 15 AUC Day 21/22 Induction of clearance Once weekly (n=3) 156 ± 117 131 ± 140 141 ± 90 10% Twice weekly (n=3) 329 ± 187 117 ± 92 198 ± 321 40% Daily dosing (n=3) 412 ± 111 21 ± 36 9 ± 16 98% Stopeck et.al. Clin. Cancer Research, 2002 Salzberg et.al, Investigational New Drugs 24: 299–304, 2006) Effect of Tamoxifen (TAM) Mediated CYP3A4 Induction Letrozole Alone Letrozole + Tamoxifen ( 6 weeks & > 4 months) Dowsett, M. et al. Clin Cancer Res 1999;5:2338-2343 62 PXR Pharmacogenomics. 2008 November; 9(11): 1695–1709. Midazolam Plasma Conc. Profile Effect of CYP3A/PXR Genotypes on CYP3A Induction 3 ID: 1 7 ID: 3 3 ID: 4 ID: 5 7 30 10 Midazolam Conc. (ng/ml) 30 ID: 6 ID: 7 ID: 8 ID: 9 ID: 10 ID: 11 ID: 12 ID: 14 10 30 10 30 ID: 15 Time(hrs) 10 3 7 Day 0 Day 1 Day 42 64 Inhibition of Drug Metabolizing Enzymes Inhibitor present Inhibitor absent CYP3A CYP3A Active drug Active drug Inactive drug Inhibitor Inactive drug Saquinavir + Ritonavir AIDS. 1997 Mar 15;11(4):F29-33 Saquinavir Plasma Rosuvastatin concentration-time profile in the absence and presence of Darunavir/Ritonavir Before DRV/RTV After DRV/RTV Desai Lab with the UC President • Graduate Students - Rucha Sane – Niresh Hariparsad – Fang Li – Ganesh Mugundu •Former Student/Post-Doc Srikanth Nallani, Ph.D., FDA • Collaborators – Arthur Buckley, Ph.D., College of Pharmacy – Julie Nelson, Ph.D., Department of Molecular Genetics, Biochemistry and Microbiology - Elizabeth Shaughnessy, MD - Judith Feinberg, MD Brian Goodwin, Ph.D., GlaxoSmithKline – Stephen Storm, Ph.D. University of Pittsburgh - • Funding Sources - Aventis Pharmaceutical, Eli Lily & Co, Bristol Myers - Womens Health (UC), American Cancer Society - NIH, Susan G. Komen Breast Cancer Foundation Squibb