Lecture_11_2100_F11
advertisement

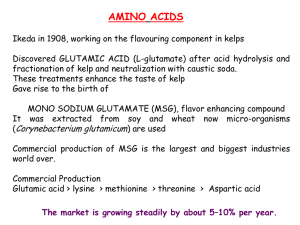
Chemistry 2100 Lecture 11 Protein Functions Binding P + L PL Catalysis Structure Why Enzymes? • • • • Higher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation COO - COO NH2 O OH COO OH COO • Metabolites have many potential pathways of decomposition Chorismate mutase COO OOC O NH2 - - O COO COO OH - • Enzymes make the desired one most favorable Specificity: Lock-and-Key Model • Proteins typically have high specificity: only certain substrates bind • High specificity can be explained by the complementary of the binding site and the ligand. •Complementarity in – size, – shape, – charge, – or hydrophobic / hydrophilic character •“Lock and Key” model by Emil Fisher (1894) assumes that complementary surfaces are preformed. + Specificity: Induced Fit • Conformational changes may occur upon ligand binding (Daniel Koshland in 1958). – This adaptation is called the induced fit. – Induced fit allows for tighter binding of the ligand – Induced fit can increase the affinity of the protein for a second ligand • Both the ligand and the protein can change their conformations + Apoenzyme + Coenzyme = Holoenzyme Apoenzyme + Coenzyme = Holoenzyme Apoenzyme + Coenzyme = Holoenzyme Apoenzyme + Coenzyme = Holoenzyme Enzymatic Activity Potential Energy • increase [reactant] • increase temperature • add catalyst Reactants Products Reaction TS Potential Energy • increase [reactant] • increase temperature • add catalyst Reactants Products Reaction TS • increase [reactant] Potential Energy Ea • increase temperature • add catalyst Reactants Products Reaction TS • increase [reactant] Potential Energy Ea • increase temperature • add catalyst Reactants Products Reaction TS • increase [reactant] Potential Energy Ea • increase temperature • add catalyst Reactants Products Reaction TS • increase [reactant] Potential Energy Ea • increase temperature • add catalyst Reactants Products Reaction TS • increase [reactant] Potential Energy Ea • increase temperature Ea' • add catalyst Reactants Products Reaction How to Lower Enzymes organizes reactive groups into proximity G ? How to Lower G ? Enzymes bind transition states best Potential Energy H2O + CO2 HOCO2– H2O + O C O O HO Reaction C O– + H+ + H+ Potential Energy H2O + CO2 H2O + O C O Reaction HOCO2– + H+ Potential Energy H2O + CO2 HOCO2– + H2O + O C O O HO Reaction C O– + H+ H+ Potential Energy H2O + CO2 HOCO2– + H2O + O C O O HO Reaction C O– + H+ H+ H2O + CO2 HOCO2– H Potential Energy H O + O C O H2O + O C O O HO Reaction C O– + H+ H+ H2O + CO2 HOCO2– H H O + O C O Potential Energy Ea H2O + O C O O HO Reaction C O– + H+ H+ H2O + CO2 HOCO2– H H O + O C O Potential Energy Ea Ea' H2O + O C O O HO Reaction C O– + H+ H+ s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase s u crose + s u crase [ s u crose-s u crasecomple x ] H2O glu cose + fru ctos e + s u crase How to Do Kinetic Measurements Enzyme Activity Figure 23.3 The effect of enzyme concentration on the rate of an enzyme-catalyzed reaction. Substrate concentration, temperature, and pH are constant. Enzyme Activity Figure 23.4 The effect of substrate concentration on the rate of an enzyme-catalyzed reaction. Enzyme concentration, temperature, and pH are constant. Enzyme Activity Figure 23.5 The effect of temperature on the rate of an enzyme-catalyzed reaction. Substrate and enzyme concentrations and pH are constant. Enzyme Activity Figure 23.6 The effect of pH on the rate of an enzyme-catalyzed reaction. Substrate and enzyme concentrations and temperature are constant. What equation models this behavior? Michaelis-Menten Equation O (C H3 ) 3 N C H2 C H2 ace tylcholine O C C H3 + H2 O AChE O (ACh) (C H3 ) 3 N C H2 C H2 c holine (Ch) OH + HO C C H3 ace tic acid O (C H3 ) 3 N C H2 C H2 ace tylcholine O C C H3 + H2 O AChE O (ACh) (C H3 ) 3 N C H2 C H2 c holine (Ch) OH + HO C C H3 ace tic acid O (C H3 ) 3 N C H2 C H2 ace tylcholine O C C H3 + H2 O AChE O (ACh) (C H3 ) 3 N C H2 C H2 c holine (Ch) OH + HO C C H3 ace tic acid O (C H3 ) 3 N C H2 C H2 ace tylcholine O C C H3 + H2 O AChE O (ACh) (C H3 ) 3 N C H2 C H2 c holine (Ch) OH + HO C C H3 ace tic acid Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O H CO OH (C H3) 3N His O C H2 C H2 O H • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2H O O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2 O • • C C H3 O N NH Ser C H2 Glu Asp CO O His O H CO OH H H (C H3) 3N C H2 C H2 O • • C C H3 O N NH O (C H3 ) 3 N C H2 C H2 ace tylcholine O C C H3 + H2 O AChE O (ACh) (C H3 ) 3 N C H2 C H2 c holine (Ch) OH + HO C C H3 ace tic acid Inhibitors • Reversible inhibitors – Temporarily bind enzyme and prevent activity • Irreversible inhibitors – Permanently bind or degrade enzyme Reversible Inhibition Irreversible Inhibition Acetylcholinesterase Ser C H2 Glu CO O Asp His O H CO OH • • N NH Ser C H2 Glu Asp CO O His O H CO OH F • • CH3 CH CH3 O P CH3 O N NH Ser C H2 Glu Asp CO O His O H CO OH F • • CH3 CH CH3 O P CH3 O N NH Ser C H2 Glu Asp CO O His O H CO OH F • • CH3 CH CH3 O P CH3 O N NH Ser C H2 Glu Asp CO O His O H CO OH F • • CH3 CH CH3 O P CH3 O N NH Ser C H2 Glu Asp CO O His O H CO OH CH3 CH CH3 O F P CH3 • • O N NH I N CH N OH CH3 Pyr idine aldoxime met hiodide ( PAM) Br Br (C H3 ) 3 N–CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 –N(C H3 ) 3 dec amet honium O (C H3 ) 3 N bromide O CH2 CH2 OCCH2 CH2 COCH 2 CH2 N(C H3 ) 3 succ inylcholine Commercial Enzymes • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays s tarch -amylase de xtrins gluc oamylase glucose gluc ose isome rase fructos e s tarch -amylase de xtrins gluc oamylase glucose gluc ose isome rase fructos e s tarch -amylase de xtrins gluc oamylase glucose gluc ose isome rase fructos e • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays • lactase • rennin • papain • high-fructose corn syrup • pectinase • clinical assays