InorgCh14.2
advertisement
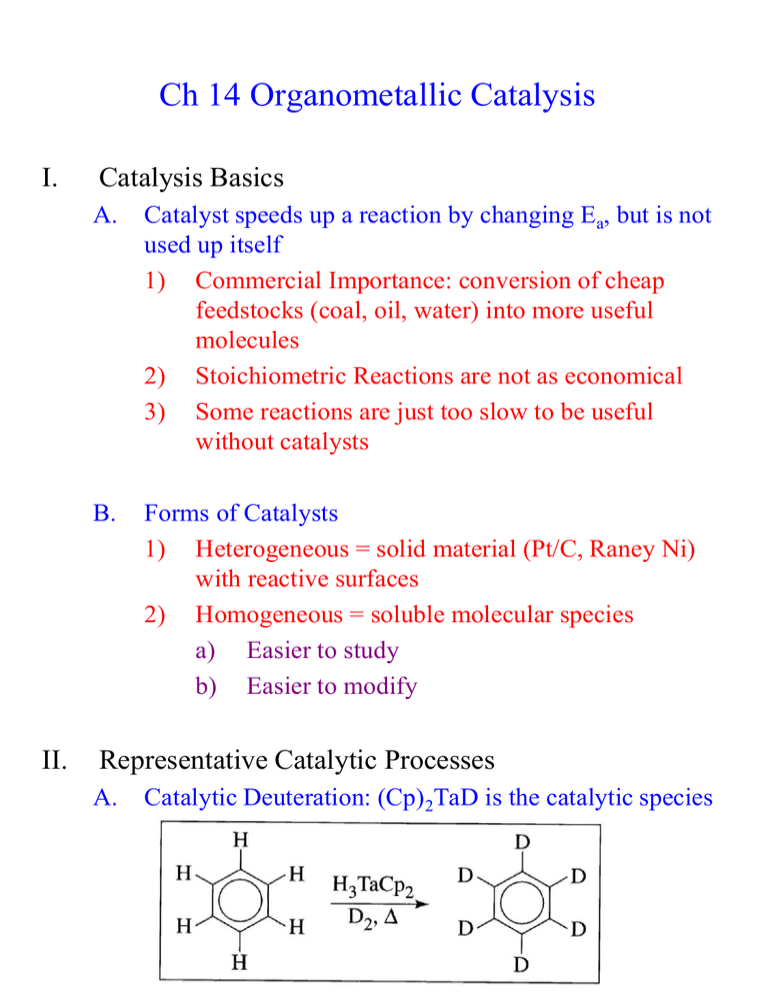
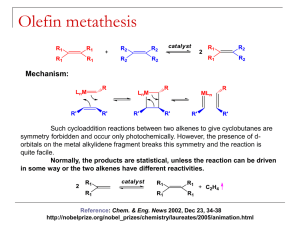
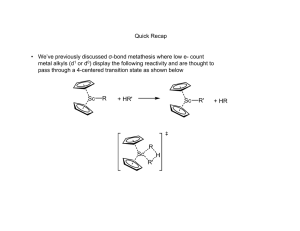
Ch 14 Organometallic Catalysis I. II. Catalysis Basics A. Catalyst speeds up a reaction by changing Ea, but is not used up itself 1) Commercial Importance: conversion of cheap feedstocks (coal, oil, water) into more useful molecules 2) Stoichiometric Reactions are not as economical 3) Some reactions are just too slow to be useful without catalysts B. Forms of Catalysts 1) Heterogeneous = solid material (Pt/C, Raney Ni) with reactive surfaces 2) Homogeneous = soluble molecular species a) Easier to study b) Easier to modify Representative Catalytic Processes A. Catalytic Deuteration: (Cp)2TaD is the catalytic species Reductive Elimination Oxidative Addition Reductive Elimination Oxidative Addition Reductive Elimination B. Hydroformylation = Oxo Process 1) Industry: CH3CH=CH2 CH3CH2CH2CHO 2) HCo(CO)3 is the catalytic species HRh(CO)2(PPh3)2 is faster, more selective catalyst for Hydroformylation Rh(I), d8, 18 e- species Dissociation of CO Rh(I), d8, 16 e- species Addition of alkene ligand Rh(I), d8, 18 e- species 1,2-Insertion Rh(I), d8, 16 e- species Addition of CO ligand Rh(I), d8, 18 e- species Alkyl Migration Rh(I), d8, 16 e- species Oxidative Addition Rh(III), d6, 18 e- species Reductive Elimination of Product, Reforming Catalyst C. Monsanto Acetic Acid Process Monsanto Acetic Acid Process: Alternative View D. Wacker (Smidt) Process Substitution Substitution 1,2-Insertion E. Hydrogenation by Wilkinson’s Catalyst Rh(I), d8, 16 e- species Rh(III), d6, 18 e- species Rh(III), d6, 16 e- species Rh(III), d6, 18 e- species Rh(III), d6, 16 e- species Rh(I), d8, 14 e- species 1) 2) Steric bulk slows down this reaction Selective for least hindered alkene E. Olefin Metathesis (Nobel Prize 2005: Grubbs, Schrock, Chauvin) 1) Metathesis = double replacement reaction H2C CHR + H2C 2) CHR CH2 CH2 + CHR CHR Several Mechanisms Have Been Proposed 3) Evidence for/against Alkyl Exchange a) b) c) 4) Reacting H3C—HC=CH—CH3 and D3C—DC=CD—CD3 =CH—CD3 and =CD—CH3 products would be expected if this mechanism was correct: it’s not None of these “mixed” products were observed “Diolefin” or “Pairwise” Mechanism a) Both alkenes (olefins) coordinate the metal ion b) A cyclobutane-like intermediate forms c) The cyclobutane decomposes to the new alkenes 5) Evidence for/against Pairwise Mechanism a) The kinetic (early) product mixture of the following reaction should contain primarily product A b) Two alkenes have to bind prior to reaction c) The thermodynamic (late) product mixture should contain a statistical mixture of A, B, and C c) 6) Result: the statistical mixture was found at all time periods (early or late), which is not consistent with this Pairwise Mechanism Carbene/non-pairwise mechanism (Chauvin Mechanism) a) A metal carbene forms first b) Only one alkene is needed to initiate reaction c) The key intermediate is a metallocyclobutane d) e) This mechanism expects an equilibrium mixture of products at all times Results above are consistent with this mechanism 7) f) Additional early/late experiments confirm: g) Carbene complex shown to undergo metathesis Schrock Metathesis Catalyst a) Most reactive (fastest) and effective b) Highly sensitive to H2O and O2, which reduces their usefulness for many reactions c) Can buy commercially d) If M = Mo and R = isopropyl = Schrock’s Catalyst 8) Grubbs I Grubb’s Metathesis Catalyst a) Less active (slower) than Schrock b) Less sensitive to water and oxygen c) Less expensive because Ru << Mo in expense Grubbs II Grubbs-Hoveyda Grubbs I: less stable, slower, still the best for some reaction Grubbs II: more stable, less sensitive to water and air, generally faster Grubb-Hoveyda: can be modified to even work in water (green chemistry) 9) Applications of Methathesis Catalysts a) Ring Closing Metathesis (RCM) b) Ring Opening Metathesis Polymerization (ROMP) 5) G. Alkyne Methathesis is also possible Heterogeneous Catalysts 1) Very important commercially 2) Mechanisms are very difficult to decipher 3) Zeigler-Natta Polymerizations a) Aluminum Alkyls + Ti catalysts polymerized olefins b) Two major proposed mechanisms appear to be valid in different cases