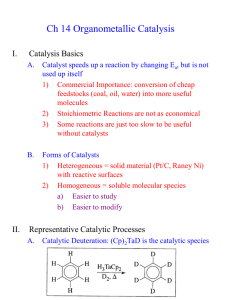
Quick Recap • We`ve previously discussed σ
advertisement
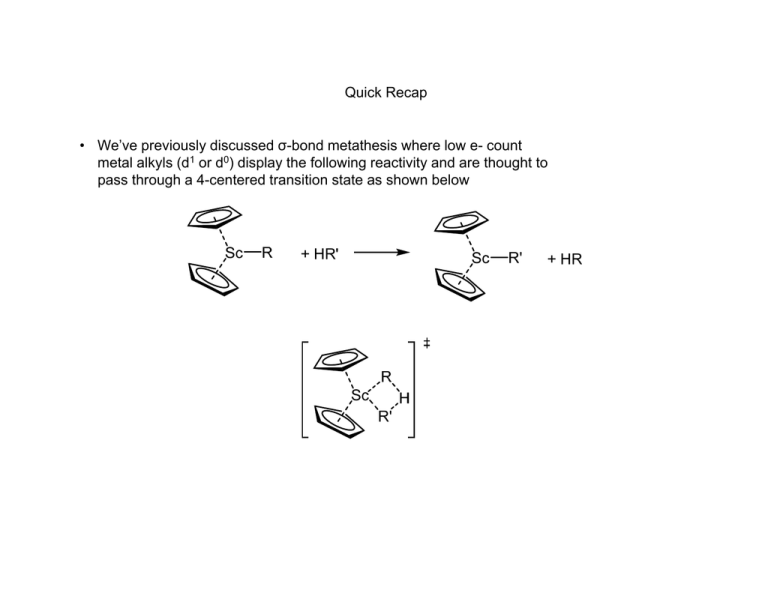
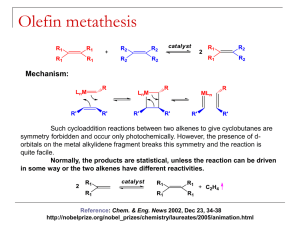
Quick Recap • We’ve previously discussed σ-bond metathesis where low e- count metal alkyls (d1 or d0) display the following reactivity and are thought to pass through a 4-centered transition state as shown below • Analogously Olefin Metathesis is R1 + R1 R2 R2 R2 [cat] 2 R1 • The reaction can be driven by pairing electron donating R1 with electron withdrawing R2 to create a stabilizing pushpull effect in the metathesis product. • Can also be driven by removal of gaseous olefins such as ethylene History Early mechanistic proposals • Bradshaw, J. Catalysis, 1967, 7, 269. Studies done on Co-Oxide/Mo-Oxides supported on alumina (CoO·MoO2·Al2O3) • Initial mechanistic proposals followed a pairwise mechanism with a cyclobutane intermediate. Such a mechanism is forbidden by Woodward-Hoffman rules for 2+2 cycloadditions. Also cyclobutanes are not detected in the reaction mixture or decomposed by the catalyst. • Alternatively a pairwise mechanism involving a metallacyclopentane was proposed. R1HC R1 M M R2HC R1 R2 R2 R1 2 1 R HC CHR M 2 R M M CH2 The Actual Mechanism • 1971 Chauvin. Proposes a sequential [2+2] cycloaddition pathway. The Actual Mechanism • 1975 Katz and McGinnis. Double cross over experiment ruled out pairwise mechanisms. Typical catalyst cocktail of MoCl2(CO)2(PPh3) and Me3Al2Cl3 • For “pairwise” mechanism we expect to see only C12 and C16 at early time points. • The C14 double-cross product should only form at early times due to a nonpairwise mechanism More Mechanistic Studies • Some people proposed that a more pairwise mechanism might still give the C14 products seen at early timepoints by Katz if the olefin was bound strongly to the metal and underwent multiple metathesis events before being released. • To disprove the “sticky olefin” hypothesis a crossover experiment where the products of metathesis cannot themselves undergo metathesis was required. • The statistical mixture of 1:2:1 d0, d2 and d4 ethylene confirms again the non-pairwise mechanism Chauvin proposed. Some Examples of Alkene and Alkyne Metathesis Reactions Catalyst Structures • In general Schrock type catalysts are based on Mo or W and Grubbs catalysts are based on Ru. Generic structures for each type are shown below. The important feature in each is the alkylidene ligand which is required for olefin metathesis activity. • In general Ru catalysts are more tolerant of functionality and have greater stability while the Mo systems display greater activity. L NAr Cl Ru Cl Mo X Ar R X L Grubbs Schrock • A number of variants have been prepared for a wide range of applications, some of which are shown below. PCy3 Cl Ru Cl Ar N N Ar Ar N Cl PCy3 Grubbs I Cl Ru Cl Ph PCy3 Grubbs II Ar F 3C Cl Ru Ph N O i Pr Hoveyda-Grubbs F 3C CF3 NAr O Mo O F3C Schrock Selectivity in Cross-Metathesis • The selectivity for the formation of different metathesis products can be predicted by the categorized olefins into four different types. • The table below shows type classifications with several examples for a Grubbs II catalyst. Type I Type II Type III Type IV Rapid homodimerization Slow homodimerization No homodimerization Spectators to cross‐ metathesis Terminal olefins, allylic alcohols, esters, allyl boronate esters, allyl halides, styrenes Styrenes w/ large ortho substituents, acrylates, vinyl ketones, perfluoroalkyl olefins 1,1‐disubstituted olefins, non‐bulky tri‐substituted olefins, vinyl phosphonates Vinyl nitro olefins, tri‐ substituted allylic alcohols (protected) • Reactions between two olefins of Type 1 leads to a statistical mixture of cross-metathesis products. • Reactions between two olefins of the same type (non Type 1) leads to non-selective cross-metathesis. • Reactions between olefins of two different types leads to selective cross metathesis. • An olefin’s type classification is somewhat dependent on catalyst identity but in general Type I olefins are unhindered and electron rich while Types II-IV are increasingly hindered and electron deficient. Mechanism of Alkyne Metathesis • Schrock has studied alkyne metathesis with • The reaction is more poorly understood using Mo(CO)6 and phenols to generate an in situ catalyst. high valent tungsten carbyne complexes and • It is possible that the Mo is oxidized and then catalyzes found it mechanistically similar to the Chauvin the reaction via the same route as the W carbyne system mechanism for alkene metathesis studied by Schrock although it is unclear how high oxidation states would be generated. • metallacyclopentadiene and butadiene complexes containing metal carbonyl fragments are known and have been proposed as relevant intermediates although supporting data is scarce. JACS, 1981, 103, 3139 JACS, 1982, 104, 6808 J. Org. Chem. 1998, 63, 8606 Mechanism of Enyne Metathesis • Mostly studied with Grubbs Ru catalsts JACS, 2005, 127, 576 Some Places You Can Go For More Information • Crabtree, Chapter 12 • Hartwig, Organotransitionmetal Chemistry, chapter 21. • https://en.wikipedia.org/wiki/Olefin_metathesis