Oxidation and Reduction Reactions
advertisement

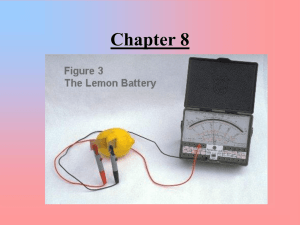
Oxidation and Reduction Reactions Oxidation (Read only) Original definition: When substances combined with oxygen. Ex: All combustion (burning) reactions CH4(g) + 2O2(g) CO2(g) + 2H2O(l) All “rusting” reactions 4Fe(s) + 3O2(g) 2Fe2O3(s) Reduction (Read Only) Original Definition: Reaction where a substance “gave up” oxygen. Called “reductions” because they produced products that were “reduced” in mass because gas escaped. Ex: 2Fe2O3(l) + 3C(s) 4Fe(l) + 3CO2(g) Oxidation/Reduction Deals with movement of ELECTRONS during a chemical reaction. (Oxygen doesn’t have to be present) Electron Transfer Reactions Oxidation: LOSS of one or more electrons. Reduction: GAIN of one or more electrons Electron Transfer Reactions Oxidation & reduction always occur together. Electrons travel from what is oxidized towards what is reduced. One atom loses e-, the other gains e- Redox Reactions: ALWAYS involve changes in charge A competition for electrons between atoms! Remember!! Or…Remember Conservation of “Charge” Total electrons lost = Total electrons gained Oxidizing/Reducing Agents Oxidizing Agent: substance reduced Gains electrons Reducing Agent: substance oxidized Loses electrons The “Agent” is the “opposite” Assigning Oxidation Numbers Practice Problems http://www.usca.edu/chemistry/genchem/oxn umb.htm Animation of Oxidation and Reduction http://www.ausetute.com.au/redox.html Identify What is Changing in Charge What is oxidized and reduced? What are the oxidizing and reducing agents? Ex: 3Br2 + 2AlI3 2AlBr3 + 3I2 0 +3 -1 3Br2 + 2AlI3 +3 -1 0 2AlBr3 + 3I2 Br2 is reduced and is the oxidizing agent I-1 is oxidized and is the reducing agent What is oxidized and reduced? What are the oxidizing and reducing agents? Mg + CuSO4 2K + Br2 Cu + 2AgNO3 MgSO4 + Cu 2KBr Cu(NO3)2 + 2Ag NOTE: Atoms in a polyatomic ion DO NOT change in charge! 0 +2 +2 Mg + CuSO4 0 MgSO4 + Cu Mg oxidized (reducing agent) Cu+2 reduced (oxidizing agent) 0 0 +1 -1 2K + Br2 2KBr K oxidized (reducing agent) Br2 reduced (oxidizing agent) 0 +1 Cu + 2AgNO3 Cu oxidized (reducing agent) Ag+1 reduced (oxidizing agent) +2 0 Cu(NO3)2 + 2Ag Redox or Not Redox (that is the question…) Redox Reactions: must have atoms changing in charge. Not all reactions are redox. Easy way to spot a redox reaction!!! Look for elements entering and leaving compounds. Is it Redox? Look for Changes in Charge! Are elements entering and leaving compounds? Synthesis: Ex: 2H2 + O2 2H2O Decomposition: Ex: 2KClO3 2KCl + 3O2 Is it Redox? Synthesis: YES 0 0 +1 -2 Ex: 2H2 + O2 2H2O Decomposition: YES +1 +5 -2 Ex: 2KClO3 +1 -1 0 2KCl + 3O2 Is it Redox? Combustion: CH4 + 2O2 CO2 + 2H20 Single Replacement: Zn + CuCl2 ZnCl2 + Cu Is it Redox? Combustion: YES -4 +1 0 CH4 + 2O2 +4 -2 CO2 + 2H20 Single Replacement: 0 +2 -1 Zn + CuCl2 +1 -2 +2 -1 YES 0 ZnCl2 + Cu Is it Redox? Double Replacement: AgNO3 + LiCl AgCl + LiNO3 Is it Redox? Double Replacement: NO!!!! Ions switch partners, but don’t change in charge +1 +5 -2 +1 -1 AgNO3 + LiCl +1 -1 +1 +5 -2 AgCl + LiNO3 Remember charges of atoms inside polyatomic ions do not change! Writing Half Reactions Redox Reactions are composed of two parts or half reactions. Half Reactions Show: Element being oxidized or reduced. Change in charge # of electrons being lost or gained Writing Half Reactions 0 0 +1 -1 2Na + F2 Oxidation: or 2NaF Na 2Na Na+1 + 1e2Na+1 + 2e- Note: e- are “lost” (on the right of arrow) Reduction: or F + 1eF2 + 2e- F-1 2F-1 Note: e- are “gained” (on the left of arrow) Ox’s Have Tails!! Oxidation Half reactions always have “tails” of electrons Na Na+1 + 1e- 0 +2 -1 +2 Zn + CuCl2 -1 0 ZnCl2 + Cu Zn+2 + 2e- Ox: Zn Red: Cu+2 + 2e- Cu Balancing Simple Redox Rxns Must be: Balanced for Mass ATOMS balance Balanced for Charge Total e- Lost = Total e- Gained Balancing Harder Redox Reactions (Honors) Oxidation Number Method (Balancing in Acid Solution) • • • • • • • • Find ox #’s and use brackets to connect elements changing in charge. Balance atoms changing in charge Find total e- involved in each change If necessary balance e- by multiplication Balance all other atoms except H and O Balance oxygen by adding H2O to side deficient Balance hydrogen by adding H+1 to side deficient Check for balance with respect to atoms and charge. Half Reaction Method (Ion/Electron Method) (In acid solution) Separate equation into two “basic” half reactions Balance all atoms except H and O Balance oxygen by adding H2O Balance hydrogen by adding H+1 Balance charge by adding electrons to more positive side If necessary balance e- by multiplication Add together half reactions and simplify Check for balance of atoms and charge Applications of Redox Reactions Corrosion of Metals the metal gets oxidized forming metal oxides on the surface Prevention: Use paint, oil, plating or attach to negative terminal of a battery. Gold doesn’t rust…Why? Photograph Development involves oxidation and reduction of silver atoms and ions Bleach acts on stains by oxidizing them, getting reduced in the process Explosives form neutral gases like N2 from compounds! Reactivity of Metals Reference Table J Metals Higher on Table J are more ‘active” It is easier for more “active” metals to be oxidized or lose electrons. Copper replaces silver! Cu0(s) + AgNO3(aq) Ag0(s) + CuNO3(aq) Ag0(s) + CuNO3(aq) wouldn’t happen!!! Reactivity of Nonmetals Reference Table J Nonmetals higher on Table J are more “active” It is easier for more “active” nonmetals to “gain” electrons and be reduced. Electrochemical Cells (Batteries) Chemical reaction that produces electricity. Called “voltaic cells” as they produce voltage This happens SPONTANEOUSLY. Moving Electrons = Electricity Electrons given off by oxidized substance travel towards substance being reduced. Traveling electrons move through “external circuit” where they do work. How do the Electrons Move? Batteries often contain 2 metals. Start with Table J Electrons travel from the more “Active metal” toward the less active metal. Metal above = oxidized Ion on Metal below = reduced Electrons flow “Down Table J” e- From metal above to ion of metal below Parts of a Simple Battery (Voltaic Cell) Made of Two “Half Cells” containing: 2 Metal Electrodes 2 Solutions of Ions External Wire Salt Bridge Electrons need to flow in a “circuit” that is connected. External Wire: allows electrons to flow between metal electrodes Salt Bridge: allows ions to flow between solutions Zn/Zn+2//Cu+2/Cu What is Ox/Red? See Table J Metal above is oxidized Zn Ion of metal below reduced Cu+2 Which way do electrons flow in the external wire? See Table J Electrons flow “Down” the table from what is oxidized towards what is reduced. from Zn to Cu e- Which electrode is negative? Which electrode is positive? Electrons flow from negative to positive electrode. eNegative electrode: Zn Positive electrode: Cu Which electrode is the anode and cathode? Anode: metal electrode where oxidation occurs Zn Cathode: metal electrode where reduction occurs Cu Remember AN OX RED CAT Anode is where oxidation happens Cathode is where reduction happens What are the Half Reactions? What is the Net Equation? Ox: Zn0 Zn+2 + 2e- e- Red: Cu+2 + 2e- Cu0 Net: (add ½ reactions) Zn0 + Cu+2 Zn+2 + Cu0 Make sure final net equation is balanced for electrons and atoms! Which electrode gains/loses weight? Look at half reactions!! Which one forms solid metal? Which one forms dissolved ions? Ox: Zn0 Zn+2 + 2eRed: Cu+2 + 2eCu0 Zinc electrode loses mass Copper electrode gains mass Which way to do the ions in the salt bridge “migrate” or move? Remember: “The negative ions complete the circuit” (The ions actually end up moving towards the solution of opposite charge that forms.) Follow the ions http://www.mhhe.com/physsci/chemistry/e ssentialchemistry/flash/galvan5.swf Dead Battery Voltage = 0 Means the reaction in the battery has reached EQUILIBRIUM. You try it… Mg/Mg+2//Al+3/Al • • • • • • • • Draw and label Battery What is oxidized/reduced? What are the half reactions and net(balanced)? What is the neg/pos electrode? What is the anode/cathode? Which way do e- flow in wire? Which way do -/+ ions flow in salt bridge? Which electrode gains/loses mass? Finding Voltage of a Battery (Honors) Use Voltage Table Find your half reactions and record voltage Note: All ½ reactions shown are reductions. For oxidation, reverse the sign of the voltage Nerntz Equation (Honors) Find voltage of a battery when the conc. of dissolved ions is not 1 Molar (as on “standard voltage” table) Ecell = E0 – 0.0592 log [product ion]x n [reactant ion]y n = total # of moles electrons being transferred The concentration of dissolved ions can affect voltage. Greater concentration of reactant ions (see net) increases the overall voltage. Electrolytic Cells & Electrolysis Reactions Uses electricity to “split” or “lyse” a compound into it’s neutral elements An outside electrical source provides electrons to force a non-spontaneous redox reaction to occur. Electrolysis Set Up Single Cell filled with electrolyte with +/- ions Attach battery to two electrodes. Electrodes are made of an inert substance (like platinum or graphite) that conducts. Electrodes don’t chemically change like in a battery, they just provide current Role of the Battery Pulls electrons off one electrode Making it POSITIVE Adds electrons onto one electrode Making it NEGATIVE Which Way do the Ions Move? To electrode of opposite charge What is Oxidized/Reduced? At neg. electrode electrons are gained by ion (reduction at CATHODE) At positive electrode electrons are lost by ion (oxidation at ANODE) Remember AN OX RED CAT Anode is where oxidation happens Cathode is where reduction happens Half Reactions & Net Equation Rxn at Anode: (Ox) ClCl + 1eOr more correctly 2ClCl2 + 2e- DIATOMIC!!!!!! Rxn at Cathode: (Red) Na+ + 1eNa (Multiply by 2 to balance electrons) NET: 2Na+ + 2Cl- 2Na + Cl2 Determining Voltage Needed Use the Voltage Table to determine the total voltage needed to run the Electrolytic cell. Total voltage should be a NEGATIVE number Electrolysis of Molten NaCl (l) Electrolysis of PbCl2(l) What is oxidized? What is reduced? What are the ox/red half reactions? Negative Electrode Positive Electrode What is the net equation? Electrolysis of PbCl2(l) Oxidized: ClHalf Reactions Ox: Cl-1 2Cl-1 Reduced: Pb+2 Cl + 1eCl2 + 2e- Red: Pb+2 + 2e- Pb Net: Pb+2 + 2Cl-1 Pb + Cl2 Electrolysis of NaCl(aq) Electrolysis of NaCl (aq) Electrolysis of Water At Positive Electrode: Ox: O-2 O + 2ebut there is a diatomic! 2O-2 O2 + 4eAt Negative Electrode Red: H+1 + 1eH but there is a diatomic! 2H+1 + 2eH2 Net: 2H2O 2H2 + O2 Lemon Battery Demo http://youtu.be/AY9qcDCFeVI Electrolysis of Copper Sulfate http://youtu.be/xBz9HJ32Ouw Electrolysis of Water/ Silver Nitrate and Cu reaction http://youtu.be/Bcfp8VtcrSA Electrolysis of Water (Animation) http://youtu.be/2t13S-KpGeE Electrolysis of Water (Simple) http://youtu.be/HQ9Fhd7P_HA Electroplating Electrolysis reaction used to coat a substance with a thin layer of metal. Often coating is a less reactive metal that is not easily oxidized or corroded. Electroplating Negative Electrode Is the OBJECT TO BE PLATED so the positive metal ions would go towards it and be REDUCED. It is the CATHODE Red: Ag+ + 1e- Ag0 Electroplating Positive Electrode Made of plating metal It dissolves into solution as metal strip gets OXIDIZED. It is the ANODE This replenishes the ions for plating. Ox: Ag0 Ag+ + 1e- Electroplating Problems (Honors) Coulomb = measure of electrical charge 1 mole e- = 96,500 coulombs # coulombs = # amps x seconds Electroplating Problems (Honors) Reduction: Happens on object to be plated Look at Reduction half reaction Look at mole relationships between electrons and metal atoms. Ex: Ag+ + 1e- Ag0 Electroplating Problems (Honors) You can now answer questions regarding the amount of a substance in moles or grams that can be electroplated over a certain amount of time. Electroplating Problems (Honors) If 10 amps are run through a CuSO4 solution for 5 minutes, calculate the grams of Cu that will plate onto the spoon. We Know: 1 mole e- = 96,500 coulombs # coulombs = # amps x seconds Red: Cu+2 + 2e- 1 mole Cu = 63.5 grams Cu0 So….Let’s start here # coulombs = 10 amps x 300 seconds = 3000 coulombs 3000 coul. x 1 mole e- x 1 mole Cu x 63.5g Cu = .987 grams 96,500 coul 2 mole e1 mole Cu Mole ratio from Reduction half reaction You Try One How long will it take to deposit 20 grams of silver from a solution of AgCl onto a copper tray if a current of 5 amps is used? Answer = 3, 574 sec or 59.5 minutes or about 1 hour You Try One How many amps are needed to deposit .504g. of Iron in 40 minutes by passing a current through a solution of Iron II Sulfate? Answer: .72 amps