Lecture series 7 - Civil and Environmental Engineering | SIU
advertisement
advertisement





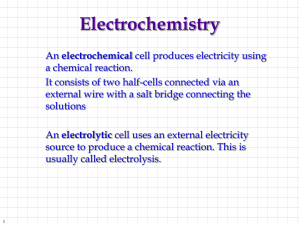
![Redox_equations[1].](http://s2.studylib.net/store/data/005611618_1-b55ee2e3a52621e48ab97cc179174553-300x300.png)



