Al(OH) 4
advertisement

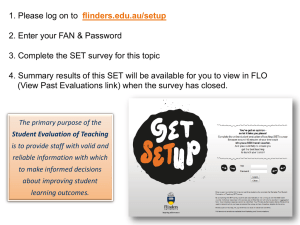
Chemistry Project Home Made Cell Chemical cell is a device in which chemical energy is converted into electrical energy. A simple chemical cell must contain 1. Two electrodes (Electrode: a metal or carbon rod that carries electricity in or out of an electrolyte during electrolysis) 2. An electrolyte (a compound which, when molten or dissolves in water (with mobile ions), conducts an electric current and is decomposed during the process) Theory of setup 1: At anode: *Al(s) + 4OH- (aq) ------> 3e- (aq) + [Al(OH)4]- (aq) (x2) At cathode: *2H+ (aq) + 2e- (aq) ------> H2 (g) (x3) Overall: [ Al(s) + 4OH- (aq) ] ,[Al(OH)4]- , H+ (aq), H2 (g) │Cu(s) From the simple electrochemical cell, as in the electrochemical series, aluminium has a higher tendency to lose electrons to form Al3+ ions. *At anode (oxidation): Al(s) ------> Al 3+ (aq) + 3e- (aq) (Impossible!!!) As aluminium (III) ions are unstable, and are difficult to form, therefore Al will react with hydroxide ions in the water to form [Al(OH)4]- which is more stable than aluminium (III) ions in water.Although the 1st I.E. of aluminium is low, from 1st I.E. onwards, the ionization power of aluminium will be decreased. From the graph, we have If we want to get one more electrons from the Al + ion, it needs to use more energy than the 1st I.E., so it is nearly impossible for aluminium to lose the 3rd electron. Therefore, at anode: Al(s) + 4OH- (aq)--------> [Al(OH)4]- (aq) + 3e- (aq) *At cathode (Reduction): Cu2+(aq) + 2e- -------> Cu(s) In the electrochemical series, Cu2+ (aq) is a stronger oxidizing agent than H+ (aq) in the electrolyte of saturated salt solution (NaCl), but the solution only contain Na+, Cl-, H+, OH-, [Al(OH)4]- but not Cu2+ ion . Therefore, hydrogen ions will be discharged at the cathode and formed on the surface of copper electrode 2H+ (aq) + 2e- (aq) --------> H2 (g) (Oxidizing Agent) As time goes by, the reaction may be stopped because the hydrogen gas bubbles formed hinder the direct contact between the copper electrode and the electrolyte. Electrolyte part : Saturated salt solution, which contains plenty of mobile ions, are used as electrolyte to complete the circuit. If the concentration of the salt solution increase, the concentration of mobile ions will also increased, as a result, the current of the electrochemical cell will also increase. e.m.f ( Electromotive force ) E total (cell) = Eθcathode – Eθanode . E total cell = Eθcathode from [ 2H+ (aq) + 2e- (aq)-->H2(g) ] - Eθanode from [ Al(s) + 4OH-(aq)--> 3e-(aq) + Al(OH)4]- (aq) ] The electromotive force of this cell is the maximum potential difference (voltage) between aluminium and hydrogen ions. The absolute potential cannot be measured directly, but we can compare them with each other Procedure of Setup 1: Chemical : Tap water 200 ml of saturated salt solution, NaCl(aq) 1x Aluminium can Apparatus: Copper wires 1 x Voltmeter Sandpapers 1x Scissors Magnetic stirrer hotplate Procedure : 1. An aluminium can and the copper strip were cut to suitable size.The inner surface of the can and the strip were cleaned by sandpaper so that aluminium oxide coated on the can,which hindered the reaction between aluminium and hydroxide ions, and copper oxide coated on the strip surface could be removed. 2. 200 ml of saturated salt solution was added into the can. 3. A circuit was connected as follow: Result Time ( min ) 0 10 20 30 40 50 60 e.m.f. ( V ) 0.30 0.30 0.40 0.40 0.40 0.35 0.35 The result table of the e.m.f. of setup 1 The e.m.f. of setup 1 e.m.f.(V) 0.50 0.40 e.m.f.(V) 0.30 0.20 0.10 0.00 0 10 20 30 40 Time(min) 50 60 70 Modifications: Although a simple cell has been set up, there are rooms of improvement. The e.m.f of the cell and the current generated by the cell are quite low. The cell should be modified so that it can be used in more electric appliances. In order to complete the circuit, the electrodes should contact with the electrolyte. The more the surface area of the electrodes contact with the electrolyte, the higher the current generated by the cell. This means the electrodes should be made as rough as possible. Beside using sodium hydroxide solution as electrolyte, we are going to add bleach in setup 2 and both bleach and sodium hydroxide (caustic soda ) in setup 3. Theory of setup 2: Bleach, containing hypochlorite ions (OCl- (aq) ), have a stronger reducing power compare with hydrogen ions ( H+ (aq) ). OCl- (aq) + H2O( l ) + 2e-(aq) <===> Cl-(aq) + 2OH-(aq) ----(*) As the difference between aluminium and hypochlorite ion in electrochemical series is greater than that between aluminium and hydroxide ion, the e.m.f. of the cell will be increased . On the other hand, the hydroxide ions produced will react with aluminium to form aluminate(IV) ions. This means the reaction will be speed up and thus increase the current generated by the cell. Procedure of Setup 2: Chemical: 1x Aluminium can 100 ml Saturated salt solution, NaCl(aq) 100ml Bleach Mercury Chloride, HgCl2 (aq) Apparatus: Copper wires 1 x Voltmeter 1 x Ammeter Sandpapers 1 x Scissors 1 x Forceps Cotton wool Magnetic stirrer hotplate Procedure: 1. An aluminium can was cut to suitable size, the inner surface was cleaned by sandpaper. The can was then washed using mercury chloride by the aid of forceps and cotton wool. 2. The copper strip was cleaned by sandpaper. The strip was bent for many times and a few cuts were made using the scissors to increase the surface area. 3. 100 ml of saturated salt solution and 100 ml of bleach was added into the can. 4. The circuit was connected as follow: Result of setup 2: Time (min) 0 10 20 30 40 50 60 e.m.f.(V) 1.1 1.2 1.0 1.0 0.9 0.9 0.8 Current (A) 0.2 0.2 0.2 0.2 0.2 0.2 0.2 The result table of e.m.f. and current of setup 2 (using saturated salt solution and bleach and sodium hydroxide) Theory of Setup 3: Sodium chloride solution, bleach and sodium hydroxide are used as electrolyte. The increase in the concentration of hydroxide ions also increases the rate of reaction with aluminium, and the current generated by cell will be increased. Moreover, refer to the equation (*), the increase in the concentration of hydroxide ions causes the equilibrium to shift to left, and more hypochlorite ions will be available for the reduction. The overall reaction is as follow: 2Al (aq) + 3OCl- (aq) + 2OH- (aq) + 3H2O(l) <===> 2[Al(OH)4]- (aq) + 3Cl- (aq) Procedure of Setup 3: 1. Repeat procedure 1-4 in setup 2, then 5 tablets (about 2g) of solid sodium hydroxide (95%) were added, so that the electrolyte of cell in setup 3 contains saturated salt solution, bleach and sodium hydroxide. Result of setup 3 Time (min) 0 10 20 30 40 50 60 e.m.f. (V) 1.4 1.2 0.3 0.2 0.1 0.1 0.0 Current (A) 0.20 0.25 0.20 0.20 0.10 0.05 0.00 The result table of e.m.f. and current of setup 3 (using saturated salt solution ,bleach, sodium hydroxide as electrolyte) The comparsion of the current between setup 2 and 3 Current(A) 0.30 0.25 Setup2 0.20 Setup3 0.15 0.10 0.05 0.00 0 10 20 30 40 Time(min) 50 60 70 The comparison of emf between setup 1 to 3 1.6 1.4 1.2 1.0 Setup1 e.m.f.(V) 0.8 Setup2 0.6 Setup3 0.4 0.2 0.0 0 10 20 30 40 Time(min.) 50 60 70 Precaution: 1. The size of the aluminium can be used in setup 1-3 and the surface area cleaned by sandpaper should be similar for fair comparison. 2. The volume of electrolyte in setup 1-3 should be the same for fair comparison. 3. The number of cuts and the level of bending of copper strip used in setup 2 and 3 should be similar for fair comparison of the function of sodium hydroxide. 4. In order to complete the circuit, the electrodes should contact with the electrolyte. The more the surface area of the electrodes contact with the electrolyte, the higher the current generated by the cell. This means the electrodes should be made as rough as possible. 5. Beside using sodium hydroxide solution as electrolyte, we are going to add bleach in setup 2 and both bleach and sodium hydroxide (caustic soda ) in setup 3. Limitation: 1. The home- made cell is in liquid form and it is difficult to handle it. 2. The chemicals inside are corrosive, so it is dangerous if use it carelessly. 3. The cell is so large that it is not portable. 4. Aluminium reacts with oxygen in air very easily, so the cell cannot be leave in air for a long time.The voltage of the cell cannot be estimated.The aluminium can will become thinner and thinner as Al(s) is oxidized to [Al(OH)4]- . So it cannot be used for a long time. Conclusion: From the result, we can see that, Setup 1, with saturated sodium chloride solution only as electrolyte, has generated stable but low e.m.f. that it is not suitable to be used in most of the electrical appliances. Setup 2, with saturated sodium chloride solution and bleach as electrolyte, has generated a quite high and stable e.m.f. It does not release so much heat and the reaction is not very violent. Setup 3, using saturated sodium chloride solution, bleach and sodium hydroxide as electrolyte, has given the highest e.m.f. among the three setups. But the e.m.f. has dropped sharply after all the NaOH(aq) had reacted. It was because the NaOH(aq) added could speed up the reaction, thus increased the e.m.f. generated. On the other hand, the disadvantage is that the life of the cell would be shortened, and the leakage of the can would be sped up. Therefore, we can conclude that the cell using sodium chloride solution and bleach as electrolyte is the best. Group Members Lau Ka Yan Connie (14) Lee Po Man Andrew (15) Li Heung Wing Henry (18) Or Ming Kuen Walter (22) Wu King Fung Ken (32)