Lecture2-amino acids acidity
advertisement

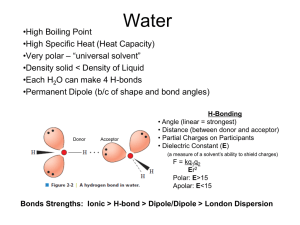
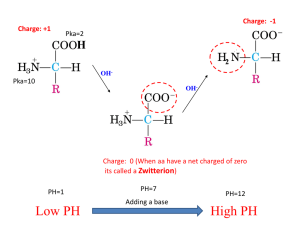
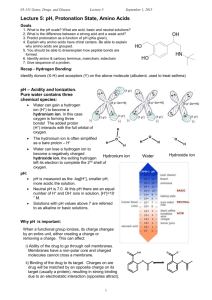
Amino acids as amphoteric compounds • • • • Acidity Basicity pKa Electronic and structural features that influence acidity and basicity General Structure of Amino Acidblocks of proteins Building • • Carboxylic acid group • Amino group • Side group R gives unique characteristics Amino acids are polar • Due to presence – polar covalent bonds – N, O and H atoms - are capable to form hydrogen bonds with water – Carry charges COO- and NH3+ The water solubility of amino acids vary to some extend, depending of side chain Carries positive charge when pH<6 Learning Check • Classify the following amino acids as hydrophobic (nonpolar), hydrophilic (polar, neutral), acidic, or basic: A. Lysine (polar basic) C. Serine (polar neutral) B. D. Leucine (nonpolar) Aspartate (polar acidic) Can act as acid (proton donor) and base (proton acceptor) The structure is dependent on pH – due to presence -COOH and -NH2 • R – COOH R – COO- + H+ acid conjugate base + R – NH + H+ • conjugate R – NH 3 2 acid base pKa of –COOH [1.8-4.3], therefore at pH 7 is COOpKa of –NH2 [9.1-12.5], therefore at pH 7 is NH3+ Zwitterion • At a particular pH, the amino acid carries no net charge and is called a zwitterion. • Zwitterion …. dipolar ion – has 1 positive and 1 negative charge • Amphoteric (ampholytes) • pH, at which the amino acid has a net charge of zero is called the isoelectric point (pI), • At the isoelectric point (pI), the + and – charges are equal. pH and ionization (1) In solutions more basic than the pI, the —NH3+ in the amino acid donates a proton and become (-NH2) . In solution more acidic than the pI, the COO- in the amino acid accepts a proton and become (-COOH). OH– H+ + H3N–CH2–COOH + H3N–CH2–COO– H2N–CH2–COO– Positive ion zwitterion Negative ion Low pH neutral pH High pH By rearranging the above equation we arrive at the Henderson-Hasselbalch equation: pH = pKa + log[A-]/[HA] The Henderson-Hasselbalch Equation At the point of the dissociation where the concentration of the conjugate base [A-] = to that of the acid [HA]: pH = pKa + log[1] The log of 1 = 0. Thus, at the mid-point of a titration of a weak acid: pKa = pH The term pKa is that pH at which an equivalent distribution of acid and conjugate base (or base and conjugate acid) exists in solution. [R - COO-] [H+] ; KCOOH= [R - COOH] KNH3+ [R - NH2] [H+] = [R - NH3+] pKa of –NH2 [9.1-12.5] pKa of –COOH [1.8-4.3] • For an amino acid with only one amine and one carboxyl group, the pI can be calculated from the mean of the pKa of this molecule: pI = (pKa1 + pKa2)/2 Leucine: pI = pKCOOH + pK NH3+ 2 2,36 + 9,6 = = 5,98 2 pH and Ionization (2) • Acidic amino acids such as aspartic acid have a second carboxyl group that can donate and accept protons. • If there were three titratable groups or other dissociating side chain groups, the pI equation would involve all three pKa's and the denominator would be "3“ pI = (pKa1 + pKa2 + pKa3)/3 • The pI for aspartic acid occurs at a pH of 2.8 Learning Check Glu ionization in water. • Indicate ionizable groups. – – – Predict ionization of this amino acid at pH=1.0 Predict ionization of this amino acid at pH=10.0 Predict ionization of this amino acid at pH=7.0 Glutamic Glu Acid pKa(COOH) pKa(NH2) pKa(R) 2.19 4.25 9.67 Peptides and Proteins Amino terminal- Carboxyl terminal- N-terminal- C-terminal Oligopeptide :a few amino acids Polypeptide : many amino acids Tetrapeptide 1. Acid-base behavior of a peptide: N-terminal, C-terminal, R-groups 2. Peptides have a characteristic titration curve and a characteristic pI value Acidity of organic compounds • Proton can be formed during break of C-H, N-H, O-H or S-H bonds. • Acidity of organic compounds increases in the following way: C-H acids < N-H acids < O-H acids < S-H acids Acidic properties • Strength of an acid depends on the stability of the formed anion. • If the formed anion is stable, it does not form the stable undissociated acid molecule and therefore there are H+ in the medium. Stability of acid anions depends on • Electronegativity of the atom to which hydrogen is attached. • Radius of the atom to which hydrogen is attached. • Delocalization of negative electric charge. Acidity and electronegativity • The more electronegative an element is, the more it helps to stabilize the negative charge of the conjugate base. • Acidity increases as the atom to which hydrogen is attached becomes more electronegative. Thus, acidity increases: CH4 < NH3 < H2O < HF (pKa values are 48, 38, 16 and 3 respectively) Basicity and and pKa values • Basicity is related to the ability of a compound to use its nonbonding electrons to combine with a proton. • A strong base has a large pKa. Basicity and electronegativity • Basicity will decrease as an atom becomes more electronegative. • Oxygen is more electronegative than nitrogen, therefore its electrons are less likely to be donated to a proton. Basicity and electronic properties • Proton can attach to the free electron pair. • Basicity increases where electrons are not delocalizated. .. increase .. • Basic properties in.. the row: S-H < O-H < N-H Delocalization effects • Delocalization of charge in the conjugate base anion through resonance is a stabilizing factor and will be reflected by an increase in acidity.