PH - squ-medicine
advertisement
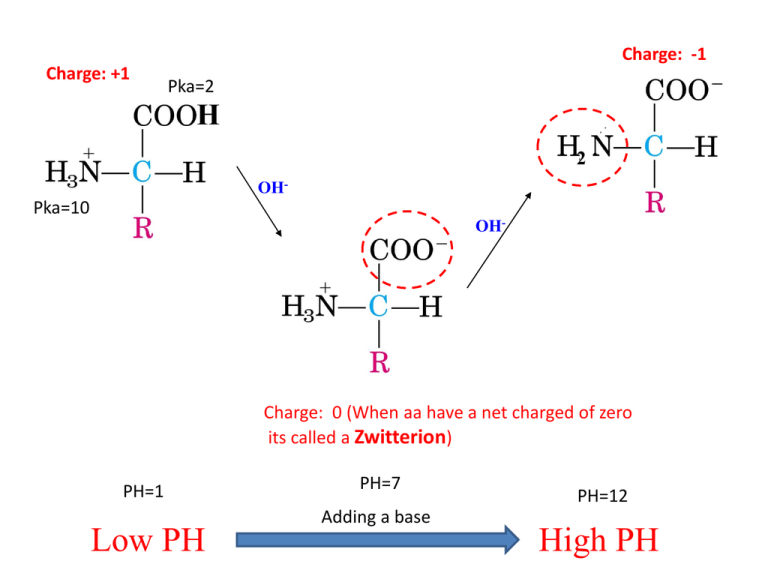
Charge: -1 Charge: +1 Pka=2 H 2 OH- Pka=10 OH- Charge: 0 (When aa have a net charged of zero its called a Zwitterion) PH=1 Low PH PH=7 Adding a base PH=12 High PH 9.60 PH/ increasing OH Pka (Low will lose first) Pka1 (for carboxyl H 2.34), Pka2 (for amino group H 9.60) PI is PH when aa is neutral PI (isoelectric point)= (Pka1+PKa2)/2 X 2.34 X 9.60 2.34 1. pH < pKaC Almost all monopositive form Avg. net charge +1 2. pH = pKaC Half monopositive, half isoelectric Avg. net charge = +0.5 3. pH = 1/2(pKaC + pKaN) All isoelectric form Avg. net charge = 0 4. pH = pKaN Half isoelectric, half mononegative Avg. net charge = -0.5 5. pH > pKaN Almost all mononegative Avg. net charge -1 Calculate the pI of methionine. Methionine has Ka values pKaC = 2.1 and pKaN = 9.3. pI = 1/2(pKaC + pKaN) pI = 1/2(2.1 + 9.3) pI = 5.7 WHAT ABOUT THE R Groups? Pka: 2.19 Pka: 6.97 1. PH 2. Pka (Low will lose first) 3. Pk1 (for carboxyl H 2.19), Pk2 (for amino group H 9.67), PKR (for R group H 4.25). 1. PH< PK1 (All Protonated) 2. PH> PK1 COOH COO3. PH> PKR COOHR COO4. PH>PK2 H3N+ H2N X Pka: 4.25 X Write equations for the dissociation of aspartate and calculate its pI. Pka: 9.8 Pka: 2.1 Pka: 3.9 pH = 3.0 A zwitterion is a molecule with both positive and negative charges, but with a net charge of zero. The isoelectric form is found after the first dissociation, between pKaC and pKaR pI = 1/2(pKaC + pKaR) pI = 1/2(2.1 + 3.9) pI = 3.0 •If the pH is less than the pI, the amino acid will have a net positive charge. •If the pH is greater than the pI, the amino acid will have a net negative charge. •If the pH equals the pI, the amino acid will have no net charge (this is the definition of pI.) Pka=6 H+ H+ Histidine: side chain can be a proton donor and a proton acceptor Histidine: weakly basic, but uncharged at physiological PH (7.4) PH> Pka , Lose the proton! Protein Structure Peptide: A short chain of amino acids. Polypeptides: A long chain of amino acids. Protein: A protein is a biological polymer of amino acids bonded together by peptide bonds between the carboxyl (-COOH) and amino (-NH2) groups of adjacent amino acid residues and folds into a defined three dimensional structure. peptide bond Primary Assembly Secondary Folding Tertiary Packing Quaternary Interaction PROCESS STRUCTURE Protein Structure: The different levels……………. The Primary Structure: Amino acids joined by peptide bonds! Defining the primary structure of a protein The primary structure of a designated protein is the amino acid sequence of the protein! Chemistry of peptide bond formation -α-carboxyl of one amino acid is joined to α -amino of a second amino acid (with removal of water). -Peptide bond has a partial double bond character. - It is a rigid bond that is shorter than a single bond. R groups are not involved in forming peptide bonds! Primary Assembly Secondary Folding Tertiary Packing Quaternary Interaction PROCESS STRUCTURE Protein Structure: The different levels……………. Defining the secondary structure of a protein local sub-structures in a polypeptide chain predominantly formed by the participation of hydrogen-bond Understanding the H-bond A hydrogen bond is the interaction of a hydrogen atom with an electronegative atom. Ex: nitrogen, oxygen etc. α-Helix α-helix is a right-handed spiral conformation Every N-H group of the amino group forms a hydrogen bond with the C=O group of the carboxylic acid group of an amino acid four residues earlier • Short peptides do not form α-helix • The side chains of the amino acids face outward! • Formed by the same groups that are involved in the formation of peptide bond (Amino group and carboxylic acid group)!. peptide bond • Some amino acids can disrupt the α-helix structure: -Proline: Insert a Kink in the chain. - Large numbers of charge amino acids (Glutamate, aspartate, histadine, Lysine and arginine) can also disrupt the helix by forming ionic bonds! Keratin……………….. • Keratin structure is nearly entirely α-Helical • Major component of tissue Such as hair and skin. β-sheet • β-strands connected by hydrogen bond to form a βsheet. • Less common than α-helix Types of β-sheet