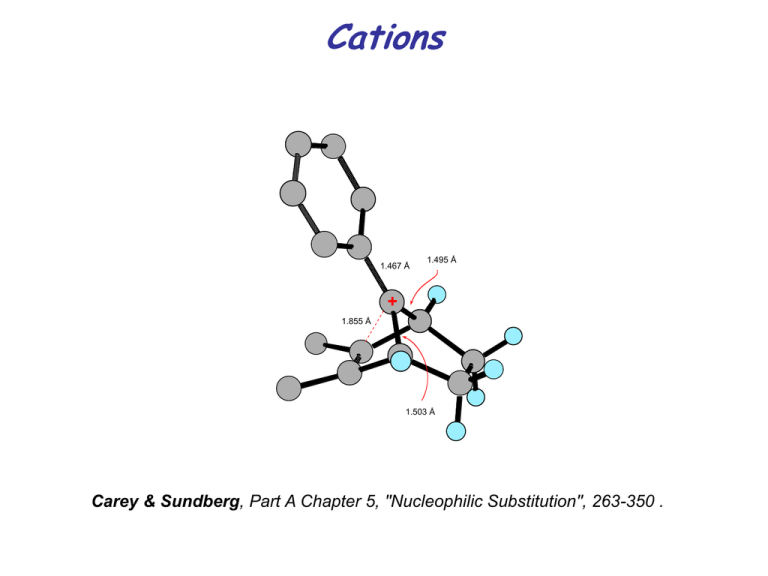
Cations
Ph
Ph
Cl
+ C
AgSbF6
Me
Me
Me
Me
F5Sb F
1.467 Å
SbF5
–
1.495 Å
+
1.855 Å
1.503 Å
T. Laube, JACS 1989, 111, 9224
Carey & Sundberg, Part A Chapter 5, "Nucleophilic Substitution", 263-350 .
John D. Roberts was born in 1918,
starting his career in 1922. He
became Prof. at MIT and then Prof.
at Caltech where he is still active.
His work is centered on mechanisms
of organic reactions.
B.A., 1941, UCLA
Ph.D. 1944, UCLA
Instructor, Harvard, 1945-6
“One of the joys of being a
professor is when an exceptional
student comes along and wants to
work with you”.
J.D. Roberts, The Right Place at the
Right Time. p. 63.
John D. Roberts graduated from the University of California at Los
Angeles where he had received A. B. (hons) degree in 1941 and the Ph.
D. degree in 1944. In 1945-1946 he was a National Research Council
Fellow and Instructor at Harvard. Later on, he went to MIT in 1946 as an
Instructor. He had introduced the terms "nonclassical" carbocations and
"benzyne" into organic chemistry. He had won numerous awards; he is a
member of the National Academy of Sciences (1956) and the American
Philosophical Society (1974). He received the Welch Award (1990, with
W. E. Doering), the National Medal of Science (1990), and the ACS
Arthur C. Cope Award (1994). Since 1939 his research has been
concerned with the mechanisms of organic reactions and the chemistry
of small-ring compounds. His current work involves applications of
nuclear magnetic resonance spectroscopy to physical organic chemistry.
Roberts made major research and pedagogic contributions to
mechanistic organic chemistry. He pioneered the use of 14C and other
isotopic labels to follow molecular rearrangements as, for example, in the
complex and subtle solvolysis of cyclopropylcarbinyl systems. He
introduced the terms "nonclassical" carbocations and "benzyne" into
organic chemistry, and used isotopic labeling to establish the
intermediacy of each. Roberts was early to recognize NMR's potential,
and used 1H NMR to study nitrogen inversion, long-range spin-spin
coupling and conformational isomerism, and later 13C and 15N NMR to
study other reactions, including the active sites of certain enzymes.
Roberts' superb short books on "Nuclear Magnetic Resonance" (1959),
"Spin-Spin Splitting in High Resolution NMR" (1961) and "Notes on
Molecular Orbital Calculations" (1961) did much to popularize and clarify
these subjects for organic chemists. His highly successful text "Basic
Principles of Organic Chemistry" (1964), written with Marjorie Caserio,
introduced spectroscopy early to undergraduates. Roberts received
many awards, including the Roger Adams (1967) and Priestley (1987)
Medals. An excellent photographer, Roberts graciously supplied several
of the photographs for the MSU collection.
Carbocations
Carbocations
+
[F5Sb–F–SbF5]–
1.431 Å
+
C
100.6 °
1.608 Å
Me
Me
Me
Q u ic k T im e ™ a n d a
T I F F ( Pa c k B it s ) d e c o m p r e s s o r
a r e n e e d e d t o s e e t h is p ic t u r e .
1.528 Å
The Adamantane Reference
(MM-2)
H
110 °
Me
1.530 Å
Me
Me
T. Laube, Angew. Chem. Int. Ed. 1986, 25, 349
Carey & Sundberg, Part A Chapter 5
Cationic Systems
Carbocation Subclasses
Carbon-substituted
R1
R1
R3
Heteroatom–stabilized
R2
R–R3 = alkyl or aryl
R3
R
O
R2
R–R3 = alkyl or aryl
R3
N
R
R2
R–R3 = alkyl or aryl
Carbocation Stability
Carbocation
Stability
Stability: Stabilization via alkyl substituents (hyperconjugation)
Order of carbocation stability: 3Þ>2Þ>1Þ
R
H
H
H
R C
> R C
> H C
> H C
R
R
R
H
Due to increasing number of substituents
capable of hyperconjugation
The relative stabilities of various carbocations
can be measured in the gas phase by their
affinity for hydride ion.
R
+ H
R–H
+ HI
Hydride Affinity = –G°
Hydride ion
affinities
CH3+
314
CH3CH2+
276
(CH3)2CH+
249
(CH3)3C+
231
+
287
386
HI increases C(+) stability decreases
H2C=CH
H
Note: As S-character increases, cation stability
decreases due to more electronegative carbon.
J. Beauchamp, J. Am. Chem. Soc. 1984, 106, 3917.
C
C+
PhCH2+
239
Carbocation
Generation
Carbocation Generation
Removal of an energy-poor anion from a neutral precursor via Lewis Acids
+
R3C X
R3C
LA
+
LA: Ag , AlCl3, SnCl4, SbCl5, SbF5, BF3, FeCl3, ZnCl2, PCl3, PCl5, POCl3 ...
X: F, Cl, Br, I, OR
LA–X
Acidic dehydratization of secondary and tertiary alcohols
- H2O
R3C OH + H–X
R3C
+
R: Aryl + other charge stabilizing substituents
X: SO42-, ClO4-, FSO3-, CF3SO3-
X
From neutral precursors via heterolytic dissociation (solvolysis) - First step in SN1 or E1 reactions
R3C X
solvent
R3C
+ X
Ability of X to function as a leaving group:
-N2+ > -OSO2R' > -OPO(OR')2 > -I • -Br > Cl > OH2+ ...
Addition of electrophiles to š-systems
R
R
R
H
R
R
H
R
R
R
R
R
H
R
R
H
Hydride abstraction from neutral precursors
R3C H
+
Lewis-Acid
R3C
R3C H
H
H
H
H
=
Lewis-Acid:
RS
RS
H
H
R2N
R2N
Ph3C BF4, BF3, PCl5
H
H
etc.
CarbocationStability
Stability
Carbocation
Vinyl & Phenyl Cations: Highly Unstable
H3C
CH2
+21
H2C
CH
HC
287
276
H2C
+81
CH
+11
C
386
Hydride ion affinities (HI)
Phenyl Cations
287
298
Allyl & Benzyl Carbocations
Carbocation Stabilization via -delocalization
Br
Stabilization by Phenyl-groups
The Benzyl cation is as stable as a t-Butylcation. This is shown in the
subsequent isodesmic equations:
Hydride ion affinities (HI)
Ph
CH2
239
Me3 C
231
Carbocations
Preparation of a vinyl cation
no good nucleophiles
prevent loss of H+
stabilizing b-Si groups
b-Si stabilization
(hyperconjugation)
Müller T., Juhasz, M., Reed, C. A., Angew. Chem. Int. Ed., 2004, 43, 1543-1546.
NMR evidence
13C
and 29Si NMR
chemical shifts
Only one 29Si signal
Symmetric in solution
(confirms ring closure)
=C+ is far downfield
Si resonance is downfield
No solvent effect
29.1
29.1
75.3
202.4
+
IR spectrum
Typical Frequencies:
B-H
C=C 1660 cm-1
C≡C 2200 cm-1
C=C+
Exp. C=C+ 1987 cm-1
Calculated: 1956 cm-1
+
CB11H6Br6-
Crystal Structure
crystal packing
Selected distances/angles
C2 - C11: 1.220 Å
C2-C11-C12: 178.8 °
Si1 – C2: 1.984 Å
Si3 – C2: 1.946 Å
Müller T., Juhasz, M., Reed, C. A., Angew. Chem. Int. Ed., 2004, 43, 1543-1546.
Cyclopropyl Cations
Cyclopropyl
Cations
Me
Carbocation Stabilization via Cyclopropylgroups
Me
C
H
A rotational barrier of about
13.7 kcal/mol is observed
X-ray Structures support this orientation
1.302 Å
1.464 Å
1.541 Å
1.409 Å
1.444 Å
O
1.222 Å
1.534 Å
1.517 Å
R
24 °
R. F. Childs, JACS 1986, 108, 1692
1.478 Å
1.474 Å
Carbocations
Inin Bridged
Bridged
Systems
Carbocations
Systems
Cyclopropyl Carbocations
Solvolysis rates represent the extend of that cyclopropyl orbital overlap
contributing to the stabiliziation of the carbenium ion which is involved as a
reactive intermediate:
OTs
Me
OTs
Cl
Me
krel = 1
krel = 1
krel = 1
OTs
OTs
Cl
krel = 106
Bridgehead Carbocations
krel = 108
krel = 10-3
Me
Me
OTs
Me
1
why so
reactive?
TsO
TsO
10-7
TsO
10-13
104
Bridgehead carbocations are highly disfavored due to a strain increase in
achieving planarity. Systems with the greatest strain increase upon passing
from ground state to transition state react slowest.
Carbocation [1,2] Sigmatropic Rearrangements
Carbocation [1,2] Sigmatropic Rearrangements
1,2 Sigmatropic shifts are the most commonly encountered cationic rearrangements. When
either an alkyl substituent or a hydride is involved, the term Wagner-Meerwein shift is
employed to identify this class of rearrangments.
Stereoelectronic requirement for migration....
retention of stereochemistry
C
D
A
C
D
A
bridging T.S.
B
B
C
D
A
B
Carbocation [1,2] Sigmatropic Rearrangements
Pinacol rearrangement (Driving force is the formation of C=O)
OH
O
H+
OH
Carbocation [1,2] Sigmatropic Rearrangements
Demjanov-rearrangement (Driving force: relief of ring strain)
Me H
Me
Me
H
H2SO4
Me
Me
Me
OH H
Me
Me
Me H
Me
H
HO
equiv to
Me
Me H
E. J. Corey J. Am. Chem. Soc. 1964, 86, 1652.
OH
Me
H
Me Me
Me
-caryophyllene alcohol
Carbocation [1,2] Sigmatropic Rearrangements
Synthesis of (±)-Isocomene
Me
Me
Me
Me
Me
Me
Me
H+
Me
Me
Me
Me
Me
Me
Me
Me
(±)-Isocumene
Pirrung, JACS 1979, 7130; 1981, 82.
The Prins Reaction
The Prins Reaction
HX
O
H
R2
+
R1
O
H
R1
H
R2
OH
R1
X-
OH
- H+
R2
OH
X
R2
R1
R2
R1
R1CHO
R2
R2
OH
R1
- H+
O
R2
O
R1
O
R2
Tandem Prins-Pinacol Reaction
The Tandem Prins–Pinacol Reaction
Ph
Ph
Ph
Me
O
Me
Lewis Acid
Me
O
Me
O
Me
O
LA
Me
Me
Prins
O
Me
Ph
Me
Me
LA
O
Me
Me
Me
O
O
LA
Me
Me
Me
pinacol
O
Ph
Me
Me
Me
O
Me
Overman’s Laurenyne
Cl
Synthesis
O
Me
(-)-Laurenyne
JACS, 1988, 110, 2248
OTBDPS
TMS
TMS
Cl
EtO
Cl
OR
OR
O
PPTS (cat.), CH2Cl2
HO
OEt
TBDPSO
Cl
1. SnCl4 (2 equiv.), 0 °C, CH2Cl2
2. TBAF
OR
O
HO
PPTS =
TBDPS = (tert-butyldiphenylsilyl)
N
H
OTs
Si
TBAF = (tetrabutylammonium fluoride) Bu4N F
Overman’s trans-Kumausyne
AcO
Synthesis
Et
O
Br
trans-Kumausyne
O
O
OH
H
OH
O
H
OR
m-CPBA
OR
RSO3H, rt
O
H
OR
4:1 regioselectivity
O
O
H
H
1.
1. Protecting Group Removal
2. Oxidation
SiMe3
Me
O
HO
DIBAL
-78 °C
H
O
O
H
OSiR3
BF3•OEt2
-78 °C rt
O
H
CHO
Et
O
Et
O
O
H
OSiR3
O
H
2. TBSCl
AcO
JACS, 1991, 113, 5378
Et
O
Br
Overman’s trans-Kumausyne
AcO
Synthesis
Et
O
Br
trans-Kumausyne
O
OH
OH
H
OH
OR
H
O
HH
OR
H
O
O
H
HO
O
OR
HO
OR
O
H
Pinacol
H
H
OR
H
O
OR
Prins
HO
O
RSO3H, rt
H
H
H
H
O
OR
The b-Silicon Effect
The b-Silicon Effect
Allyl– & Vinylsilanes react with electrophiles
E
R3Si
SiMe3
E
E
E
"R3Si+"
"R3Si+"
Mechanism - the simple picture: b-Silicon stabilizes the carbocation
E
R3Si
SiMe3
E
Nu
R3Si
E
H2C
Nu
SiMe3
E
E
E
b-Silicon EffectEffect
The The
b-Silicon
b-Silicon Effect: the origin of regioselectivity
E
occ
Si
pz
pz
Si–C pz empty
SiC
H3Si
A
H
H3 C
versus
CH2
H
H
B
CH2
H
Calculation: A more stable than B by 38 kcal/mol.
Jorgensen JACS 1985, 107, 1496.
Magnitude of the b-Silicon Effect
SiMe3
Me3C
H
H
H
Me
Solvolysis (CF3CH2OH)
OCOCF3
1
k1
k2
Me3C
= 2.4 x 10+12
H
H
2
H
Me3C
H
H
OCOCF3
H
SiMe3
OCOCF3
3
H
Solvolysis (CF3CH2OH)
k3
k4
Me3C
= 4 x 10+4
H
H
4
Me
OCOCF3
"These figures established the b-effect as one of the kinetically
strongest in organic chemistry": J. Lambert
Reactionsof
of Allylsilanes
Reactions
Allylsilanes
Allylsilanes add to aldehydes and acetals under Lewis acid promotion
OH
O
Me3Si
Ph
+
H
TiCl4
n-C3H7
Me
Ph
OH
O
Me3Si
+
TiCl4
H
Ph
n-C3H7
Me
Ph
Felkin Selectivity also holds with this class of nucleophiles
Acetals can be used as well
Me3Si
OCH3
Me
+
Me
H3CO
n-C4H9
OCH3
Me3Si
+
Me Me
H3CO
n-C4H9
OCH3
TiCl4
n-C4H9
(80%)
TiCl4
Me Me
OCH3
Me
n-C4H9
(83%)
Me
The Sakurai Reaction (Enone Conjugate Addition)
O
OTiCl4
Me
O
TiCl4
Me
Me3Si
Me
75%
CH2Cl2
SiMe3
Me
Me3Si
Fleming, Org. Reactions 1989, 37, 127-133
17%
O
regioselectivity: Allyl inversion
Iminium
Ions
Iminium Ions
R3
R1
N
R2
X-
R4
Common Methods of Generation:
R1
O
H N
R3
OR2
or Lewis Acid
R4
R2
H+, -ROH
N
N R1
or Lewis Acid
Oxidation of Amines
Hg
R1
X–
Hg(0)
X
Me
HgX2
N
R3
R1
N
R4
R2
H+, -H2O
N
H Me
Me
X–
N
rds
H
H
HX
Iminium Ions
Iminium Ions
Ph
TFA
Me3Si
Ph
N
(Z)
rel rates: 7000/1
N
Ph
H
H
TFA
(E)
N
Overman et al. TL 1984, 25, 5739.
Me3Si
Ph
Ph
SiMe3
N H
H
N H
H
H
SiMe3
(Z) vinylsilane)
H
(E) vinylsilane)
Only in the case of the (Z) vinylsilane is the emerging p orbital coplanar with CSi bond. Full stabilization of the empty orbital cannot occur with the (E)
vinylsilane.....hence the rate difference.
N-Acyliminium
Rearrangements
N-Acyliminium
Ion Ion
Rearrangements
BnO
BnO
OAc
O
O
OH
OAc
NaBH4,
Me
N
OH
N
Me
Me
MeOH,
Me
OBn
H
N
Me O
Me
O
OAc
O
[3,3]
O
BnO
H
Me
N
H
(-)-hastancine
O
OAc
Me
Me
O
HO
H
H
Me
HCO2
BnO
OAc
OBn
N
Me
OAc
N
Me O
O
H
OH
Synthesis of (-)-hastanecine: Hart JOC 1985, 50, 235.
N
The Aza-Cope-Mannich Reaction Sequence
Aza-Cope
Manich Reactions
CH2O, Na2SO4
N
H
OR
OR
OR
OR
MeCN, 80ÞC
HO
HO
HO
HO
N
[3,3]
N
N
NR2
NR2
NR2
NR2
OR
ROH2C
N
N
O
NR2
OR
HO
O
NR2
Axial Attack
N
H
N
O
Overman et al. JACS 1995, 117, 5776.
H
HO
strychnine
N
Mannich
Rxn
NR2
Terpenes
Terpenes - natural products whose carbon skeletons are built up largely from isoprene subunits:
Me
Me
Me
Me
O
O
H
H
Me
(S)-carvone
caraway
Me
(R)-carvone
spearmint
OH
isoprene
Me
Me
menthol
Me
Me
Me
Me
H
Me
H
H
O
nepetalactone
oil of catnip
OH
chrysanthemic
acid
O
(an insecticide)
O
O
H
O
O
Me
Me
Me
H2C
Me
Me
Me
periplanone
sex attractant pheromone of the
American cockroach
nootkatone
grapefruit flavor
H
Me
H
O
Me
Me
Me
H
Me
Me
HO
Me
H
Me
cholesterol
steroid
hormones
Me
O
Me
Me
citronellal
lemon oil
H
IsopreneIsoprene
: Nature's
C5 Building
Block
: Nature's Building
Block
Classification of terpenes
Me
monoterpenes : 10 C-atoms (2 isoprene units)
sesquiterpenes : 15 C-atoms (3 isoprene units)
diterpenes
: 20 C-atoms (4 isoprene units)
triterpenes
: 30 C-atoms (6 isoprene units)
tail
head
2-methyl-1,3-butadiene
isoprene
t
h
OH
OH
t
O
OH
h
geraniol
citronellol
menthol
camphor
natural rubber
ß-carotene
n
Terpene Biosynthesis
Biosynthesis
Terpene
Two isoprene units are used to build terpenes:
Me
Me
enzyme
OX
Me
-dimethylallyl pyrophosphate
(DMAP)
isopentenyl pyrophosphate
(IPP)
O
O
O
R O P O P OH
O
OX
ROX
R O S
CH3
ROTs
O
O
pyrophosphate:
nature's leaving group
tosylate:
chemist's leaving group
The general reaction process: alkene addition to electrophiles:
Me
Me
Me
OX
Me
Me
-OXMe
CH2
Me
Me
-HB
OX
Me
OX
Me
Me
OX
H H
DMAP
geranyl pyrophosphate
BH2O/OH-
Me
OH
geraniol
Me
Me
Terpene Biosynthesis
From isoprene to pinene and bornene
Me
OX
OX
Me
OX
Me
Me
Me
Me
Me
isomerization
-H+
-OX-
-OX-
OX
Me
Me
Me
Me
Me
Me
Me
geranyl pyrophosphate
Me
Me
Me
limonene
-H+
Me
Me
Me
Me
Me
Me
1,2 shift
Me
Me
-H+
bornene
pinene
Me
Steriod
andandSqualene
Oxide
Cyclization
Biosynthesis
Squalene Oxide
Squalene
Me
Me
Me
O P P
Me
dimerization
Me
Me
Me
Me
Me
Me
farnesyl pyrophosphate
(C15)
Me
Me
squalene (C30)
epoxidation
Me
Me
Me
Me
Me
O
Me
squalene oxide
Me
Me
Steriod Biosynthesis; Squalene Oxide Cyclization
Steriod Biosynthesis; Squalene Oxide Cyclization
Me
Me
Me
Me
Me
O
Me
squalene oxide
Me
Me
Me H
H+
Me
Me
Me
O
Me
Me
Me
Me
The enzyme folds the squalene oxide
into the chair-boat-chair conformation
Me
Me
Me
Me
Me
Me
Me
H
H
Me
Me
H
Me
Me
HO
HO
Me
H
Me
Me
H
H
Me
Me
H
A series of 1,2-hydride and methyl shifts occur
elimination
Me
Me
Me
Me
Me
H
Me
Higher Steriods
HO
Me
H
Me
lanosterol