Practice Problem - HCC Southeast Commons
advertisement

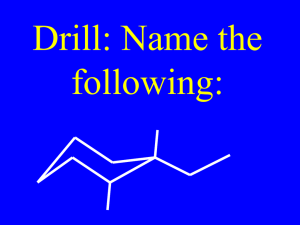
Chapter 6 Introduction • An alkene is a hydrocarbon that contains a carbon-carbon double bond It is also called an olefin but alkene is better It is abundant in nature It is an important industrial product • It includes many naturally occurring materials – flavors, fragrances, vitamins I. Alkenes: An Overview A. Industrial Preparation and Use of Alkenes B. Degree of Unsaturation C. Naming Alkenes A. Industrial Preparation and Use of Alkenes Ethylene and propylene are – the simplest alkenes – the most important organic chemicals produced industrially Compounds derived from ethylene and propylene Industrial Synthesis of Ethylene, Propylene and Butene • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of light (C2-C8) alkanes: Industrial Synthesis of ethylene, propylene and butene • The high-temperature conditions cause spontaneous homolytic breaking of C-C and C-H bonds, forming smaller fragments: • Thermal cracking is an example of a reaction whose energetics is governed by TDSo rather than DHo DGo = DHo – TDSo TDSo > DHo B. Degree of Unsaturation • Alkenes - are unsaturated hydrocarbons. • They have fewer hydrogens than alkanes with same number of carbons • Acyclic Alkanes - have the general formula CnH2n+2 where n is an integer • Acyclic Alkenes - have the general formula CnH2n where n is an integer • Degree of Unsaturation is the number of p bonds and/or rings in the molecule. • Each ring or multiple bond replaces 2 H's in the alkane formula CnH2n+2 • It relates molecular formula to possible structures • Degree of Unsaturation is the number of p bonds and/or rings in the molecule. Degree of Unsaturation = (2n+2) - x 2 where n is the number of carbons x is the number of hydrogens Example: A hydrocarbon with a molecular weight (82 g/mol) C6H10 • Saturated is C6H14 – Therefore 4 H's are not present H H H H C C H3C C C H • This has two degrees of unsaturation – – – – Two double bonds? or triple bond? or two rings? or ring and double bond? H H H CH3 Degree of Unsaturation With Other Elements 1. Organohalogens (C, H, X where X: F, Cl, Br, I) • Halogen replaces hydrogen • Add the number of halogens to the number of hydrogens • C4H6Br2 and C4H8 have one degree of unsaturation 2. Organooxygens (C, H, O) • • Oxygen forms two bonds. It does not affect the total count of H's Ignore the number of oxygens • C5H8O and C5H8 have two degrees of unsaturation 3. Organonitrogens (C, H, N) • • Nitrogen has three bonds. So if it connects where H was, it adds a connection point Subtract the number of nitrogens from the number of hydrogens Summary: Degree of Unsaturation Degree of Unsaturation can be calculated: • Count pairs of H's below CnH 2n+2 • Add number of halogens to number of H's (X equivalent to H) • Ignore or don't count oxygens (O links H) • Subtract number of nitrogens from H's (N has two connections) Degree of Unsaturation and Variation • Compounds with the same degree of unsaturation can have many things in common and still be very different Practice Problem: Calculate the degree of unsaturation in the following hydrocarbons: a. C8H14 b. C5H6 c. C12H20 d. C20H32 e. C40H56 (b-carotene) Practice Problem: Calculate the degree of unsaturation in the following formulas, and then draw as many structures as you can for each: a. C4H8 b. C4H6 c. C3H4 Practice Problem: Calculate the degree of unsaturation in the following formulas: a. C6H5N b. C6H5NO2 c. C8H9Cl3 d. C9H16Br2 e. C10H12N2O3 f. C20H32ClN C. Naming Alkenes • Like alkanes, alkenes are named according to the system devised by the International Union of Pure and Applied Chemistry (IUPAC). Steps to naming alkenes1 1. Name the parent hydrocarbon a. Find the longest continuous carbon chain containing the double bond b. Name using the suffix -ene Steps to naming alkenes2 2. Number the carbon atoms in the main chain a. The double-bond carbons receive the lowest possible numbers b. The correct sequence is when the substituents have the lowest possible number Steps to naming alkenes3 3. Write the full name a. Name, number and list the substituents alphabetically b. Indicate the position of the double-bond Steps to naming alkenes3 3. Write the full name c. If more than one double bond is present: – indicate the position of each and – Use the suffixes -diene, -triene, … Naming Cycloalkenes • Like alkenes, cycloalkenes are also named by the rules devised by the International Union of Pure and Applied Chemistry (IUPAC). Steps to naming cycloalkenes1 1. Name the parent hydrocarbon a. Number the cycloalkene so that the double bond is between C1 and C2 b. Name using the prefix cyclo- and the suffix -ene Steps to naming cycloalkenes2 2. Number the substituents and write the name • The correct sequence is when the substituents have the lowest possible number Common Names of Alkenes • Many alkenes are known by their common names. • These common names are recognized by the International Union of Pure and Applied Chemistry (IUPAC). Practice Problem: Give IUPAC names for the following compounds: Practice Problem: Draw structures corresponding to the following IUPAC names: a. 2-Methyl-1,5-hexadiene b. 3-Ethyl-2,2-dimethyl-3-heptene c. 2,3,3-Trimethyl-1,4,6-octatriene d. 3,4-Diisopropyl-2,5-dimethyl-3-hexene e. 4-tert-Butyl-2-methylheptane Practice Problem: Name the following cycloalkenes: II. Alkenes: Structure A. Electronic Structure B. Cis-Trans Isomerism C. Sequence Rules: The E, Z Designation D. Stability of Alkenes A. Electronic Structure of Alkenes • Carbon atoms in a double bond are sp2-hybridized – Three equivalent orbitals are in a plane at 120º angle – Fourth orbital is atomic p orbital (perpendicular to the plane) Two sp2-hybridized Carbon atoms form: • bond – Head-on overlap of two sp2 orbitals forms a bond • p bond – Side-to-side overlap of two p orbitals forms a p bond Molecular Orbitals (MO) • Additive interaction of p orbitals (combining p orbital lobes with the same algebraic sign) creates a p bonding orbital • Subtractive interaction (combining lobes with opposite signs) creates a p anti-bonding orbital • Only bonding MO is occupied. • Occupied p orbital prevents rotation. – Rotation prevented by p bond - high barrier, about 268 kJ/mole in ethylene Rotation of p Bond is Prohibitive • The p bond does not have circular cross-section. • The p bond must break for rotation to take place around a carbon-carbon double bond (unlike a carbon-carbon single bond). • It creates possible alternative structures B. Cis-Trans Isomerism • The presence of a carboncarbon double can create two possible structures – cis isomer - two similar groups on same side of the double bond – trans isomer - similar groups on opposite sides Cis-Trans Isomerism requires that end groups must differ in pairs Each carbon must have two different groups for these isomers to occur Cis-Trans Isomerism requires that end groups must differ in pairs • 180°rotation superposes the upper pair • Bottom pair cannot be superposed without breaking C=C X Practice Problem: Which of the following compounds can exist as pairs of cis-trans isomers? Draw each cistrans pair, and indicate the geometry of each isomer. a. CH3CH=CH2 b. (CH3)2C=CHCH3 c. CH3CH2CH=CHCH3 d. (CH3)2C=C(CH3)CH2CH3 e. ClCH=CHCl f. BrCH=CHCl Practice Problem: Name the following alkenes, including the cis or trans designation: C. Sequence Rules: The E, Z Designation • Neither compound is clearly “cis” or “trans” – Substituents on C1 are different than those on C2 – We need to define “similarity” in a precise way to distinguish the two stereoisomers • Cis-trans nomenclature only works for disubstituted double bonds Develop a System for Comparison of Priority of Substituents • Assume a valuation system – If Br has a higher “value” than Cl – If CH3 is higher than H • Then, in A, the higher value groups are on opposite sides • In B, they are on the same side – Requires a universally accepted “valuation” E,Z Stereochemical Nomenclature • Priority rules of Cahn, Ingold, and Prelog are used. – Compare where higher priority group is with respect to C=C bond and designate a prefix – E - entgegen: opposite sides – Z - zusammen: together on the same side E - entgegen Z - zusammen Cahn-Ingold-Prelog Rules 1. Ranking Priorities a. Look at the atoms directly attached to each double-bond carbon b. Rank them according to atomic number. Higher atomic number gets higher priority. In this case, the higher priority groups are opposite: (E )-1-bromo-1-chloro-propene Cahn-Ingold-Prelog Rules 2. Extended Comparison a. If atomic numbers are the same for the first atoms, compare the second, third, or fourth atoms away from the double bond carbons b. Compare until something has higher atomic number c. Do not combine – always compare Cahn-Ingold-Prelog Rules 3. Dealing With Multiple Bonds a. Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. • Substituent is drawn with connections shown and no double or triple bonds • Added atoms are valued with 0 ligands themselves Practice Problem: Which member in each of the following sets has higher priority? a. -H or -Br b. -Cl or -Br b. -CH3 or -CH2CH3 c. -NH2 or -OH d. -CH2OH or –CH3 e. -CH2OH or –CH=O Practice Problem: Which member in each of the following sets has higher priority? a. -CH3, -OH, -H, -Cl b. -CH3, -CH2CH3, -CH=CH2, -CH2OH c. -CO2H, -CH2OH, -C≡N, -CH2NH2 d. -CH2CH3, -C≡CH, -C≡N, -CH2OCH3 Practice Problem: Assign E or Z configuration to the following alkenes: Practice Problem: Assign stereochemistry (E or Z) to the following alkene, and convert the drawing into a skeletal structure (red = O) D. Stability of Alkenes • Cis alkenes are less stable than trans alkenes. – This is due to steric (spatial) strain between the two bulky substituents on the same side of the double bond < Determination of Relative Stabilities of Alkenes The relative stabilities of alkenes can be determined by: 1. establishing a cis-trans equilibrium through treatment with strong acid 2. comparing the heats of combustion (DHocombustion) for the two isomers 3. comparing the heats of hydrogenation (DHo hydrogenation) for the two isomers Less stable isomer is higher in energy and gives off more heat; More stable isomer is lower in energy and gives off less heat. 1. Cis-trans equilibrium through treatment with strong acid • After treatment of 2-butene with strong acid, at equilibrium, the trans isomer is more favored than the cis isomer by a ratio of 76 to 24 : • Cis-2-butene is less stable than trans-2-butene by 2.8 kJ/mol DE = - RT ln K 2. Heats of combustion (DHocombustion) • Comparing the heats of combustion of the two isomers of 2butene indicates that cis-2-butene is more strained than trans-2-butene by 3.3 kJ/mol: 3. Heats of hydrogenation (DHohydrogenation) • Alkenes undergo a hydrogenation reaction on treatment with H2 gas in the presence of a catalyst (Pd or Pt): • More energy is released in the hydrogenation of the cis isomer than the trans isomer (DGocis > DGotrans) because the cis alkene has a higher energy level. • The energy difference between the 2-butene isomers as calculated from the heats of hydrogenation (DHohydro) is ~ 4kJ/mol and is in agreement with the other two methods. – Trans-2-butene generates 4 kJ/mol less heat than cis-2-butene Alkenes become more stable with increasing substitution: Stabilities of Alkenes As a general rule, alkenes follow the stability order: This order of stability is due to: • hyperconjugation • bond strength Hyperconjugation Hyperconjugation - is a stabilizing interaction between the unfilled antibonding C=C p bond orbital and a filled C-H bond orbital on a neighboring substituent The more substituents, the more opportunities exist for hyperconjugation and the more stable the alkene • Electrons in neighboring filled orbital stabilize vacant antibonding p orbital – net positive interaction • Alkyl groups are better than H Bond Strength • A bond between an sp2 carbon and an sp3 carbon is stronger than a bond between two sp3 carbons. sp2-sp3 > sp3-sp3 • More highly substituted alkenes always have a higher ratio of sp2-sp3 bonds to sp3-sp3 bonds than less highly substituted alkenes and thus are more stable Practice Problem: Name the following alkenes, and tell which compounds in each of the following pairs are more stable: III. Alkenes: Reactivity A. Electrophilic Addition of HX to Alkenes B. Orientation of Electrophilic Addition: Markovnikov’s Rule C. Carbocation Structure and Stability III. Alkenes: Reactivity D. The Hammond Postulate E. Carbocation Rearrangements A. Electrophilic Addition of HX to Alkenes • General reaction mechanism of electrophilic addition: – Attack on electrophile (such as HBr) by a p bond of alkene (nucleophile) – This produces carbocation and bromide ion – Carbocation is an electrophile, reacting with nucleophilic bromide ion Mechanism: electrophilic addition of HBr to 2-methylpropene • Addition of hydrogen bromide to 2-Methylpropene • H-Br transfers proton to C=C • Forms carbocation intermediate – More stable cation forms • Bromide adds to carbocation Electrophilic Addition Energy Path • Two step process • First transition state is high energy point Energy Diagram for Electrophilic Addition • Rate determining (slowest) step has highest energy transition state – Independent of direction – In this case it is the first step in forward direction – “rate” is not the same as “rate constant” Electrophilic Addition for preparations • The reaction is successful with HCl and with HI as well as HBr • Note that HI is generated from KI and phosphoric acid Writing Organic Reactions • • • • • No established convention – shorthand Can be formal kinetic expression Not necessarily balanced Reactants can be before or on arrow Solvent, temperature, and details are on arrow • Reactants can be before or on arrow • Solvent, temperature, and details are on arrow B. Orientation of Electrophilic Addition: Markovnikov’s Rule • In an unsymmetrical alkene, HX reagents can add in two different ways, but one way may be preferred over the other • If one orientation predominates, the reaction is regiospecific Markovnikov’s Rule • In the 19th century, Markovnikov observed that: In the addition of HX to alkene, the H attaches to the carbon with the most H’s and X attaches to the carbon with the most alkyl substituents – This is Markovnikov’s rule Examples of Markovnikov’s Rule • Addition of HCl to 2-methylpropene • Regiospecific – one product forms where two are possible • If both ends have similar substitution (the same degree of substitution), then not regiospecific Markovnikov’s Rule (Restated) • Markovnikov’s rule can be restated: In the addition of HX to alkene, the more highly substituted carbocation is formed as the intermediate rather than the less highly substituted one. Energy of Carbocations and Markovnikov’s Rule • More stable carbocation forms faster • Tertiary cations and associated transition states are more stable than primary cations Mechanistic Source of Regiospecificity in Addition Reactions • If addition involves a carbocation intermediate – and there are two possible ways to add – the route producing the more alkyl substituted cationic center is lower in energy – alkyl groups stabilize carbocation Practice Problem: Predict the products of the following reactions: Practice Problem: What alkenes would you start with to prepare the following alkyl halides? a. Bromocyclopentane b. 1-Ethyl-1-iodocyclohexane C. Carbocation Structure and Stability • Carbocations are planar • The trivalent carbon: – is sp2 hybridized – has a vacant p-orbital Structure • Carbocations are planar • The trivalent carbon is sp2 hybridized. • The tricoordinate carbon is surrounded by only 6 electrons in sp2 orbitals • The three substituents are oriented to the corners of equilateral triangle • The fourth orbital on carbon is a vacant p-orbital Stability • The stability of carbocations increases with increasing substitution: • More highly substituted carbocations are more stable than less highly substituted ones. • Therefore stability of carbocations: 3º > 2º > 1º > +CH3 • The stability of carbocation (R+) can be determined by measuring energy needed to form it from R-X: R-X R+ + :X• 3º alkyl halides dissociate to give R+ more easily than 2º and 1º Why are more highly substituted carbocations more stable than less highly substituted ones? Carbocations follow the stability order: This order of stability is due to: • Inductive effects • hyperconjugation Inductive Effects Inductive Effects – result from shifting of electrons in a bond in response to the electronegativity of nearby atom – Electrons from a relatively large and polarizable alkyl group can shift toward neighboring positive charge more easily than the electron from H – The more alkyl groups attached to positively charged carbon, the more electron density shift and the more inductive stabilization Hyperconjugation Hyperconjugation - is a stabilizing interaction between a vacant p orbital and a properly oriented neighboring C-H bond. – The more alkyl groups there are on the carbocation, the more possibilities there are for hyperconjugation and the more stable the carbocation Practice Problem: Show the structures of the carbocation intermediates you would expect in the following reactions: Practice Problem: Draw a skeletal structure of the following carbocation. Identify it as primary, secondary, or tertiary, and identify the hydrogen atoms that are involved in hyperconjugation in the conformation shown: D. The Hammond Postulate • Electrophilic addition to an unsymmetrically substituted alkene gives the more highly substituted carbocation: – A more highly substituted carbocation forms faster and rapidly goes to the final product. – A more highly subsbtituted carbocation is more stable than a less substituted one Why does the stability of the carbocation intermediate affect the rate of its formation? – The relative stability of the carbocation intermediate is determined by DGº • It is related to an equilibrium constant – The reaction rate is determined by DG‡. • The relative stability of the transition state (which describes the size of the rate constant) is the activation energy (DG‡) – This is not a direct relationship In general, the faster reaction forms the more stable intermediate and the slower reaction forms the less stable intermediate Typical Transition State Structures • A transition state is the highest energy species in a reaction step – By definition, its structure is not stable enough to exist for one vibration – The transition state is transient and cannot be examined – But the structure controls the rate of reaction – Its properties may be determined in an informed way -- the Hammond Postulate Hammond’s Postulate The structure of a transition state resembles the structure of the nearest stable species – Transition states for endergonic steps structurally resemble products – Transition states for exergonic steps structurally resemble reactants Statement of the Hammond Postulate • A transition state should be similar to an intermediate that is close in energy • Sequential states on a reaction path that are close in energy are likely to be close in structure - G. S. Hammond In a reaction involving a carbocation, the transition states look like the intermediate G carbocation Reaction Competing Reactions and the Hammond Postulate • Normal Expectation: Faster reaction gives more stable intermediate • Intermediate resembles transition state “Non-Hammond” Behavior • More stable intermediate from slower reaction • Conclusion: Transition state and intermediate must not be similar in this case – not common How does the Hammond postulate apply to electrophilic addition reactions? Formation of carbocation by protonation of an alkene is an endergonic step: – The transition state is similar to carbocation intermediate – Any factor that makes the carbocation stable makes the nearby transition state more stable Transition State for Alkene Protonation • Resembles carbocation intermediate • Close in energy and adjacent on pathway • Hammond Postulate says they should be similar in structure Practice Problem: What about the second step in the electrophilic addition of HCl to an alkene – the reaction of chloride ion with the carbocation intermediate? Is this step exergonic or endergonic? Does the transition state for this second step resemble the reactant (carbocation) or product (alkyl chloride)? Make a rough drawing of what the transitionstate structure might look like. E. Carbocation Rearrangements • Carbocations undergo structural rearrangements. • A less stable carbocation rearranges to a more stable ion. • Carbocations undergo structural rearrangements following set patterns: – 1,2-hydride shift – 1,2-alkyl shift • Goes to give more stable carbocation • Can go through less stable ions as intermediates • Hydride shift – is the shift of a hydrogen atom and its electron pair (a hydride :H-) between neighboring carbons • Alkyl shift – is the shift of an alkyl and its electron pair (an alkyl anion :R-) between neighboring carbons Practice Problem: On treatment with HBr, vinylcyclohexane undergoes addition and rearrangment to yield 1-bromo-1-ethylcyclohexane. Using curved arrows, propose a mechanism to account for this result: Chapter 6