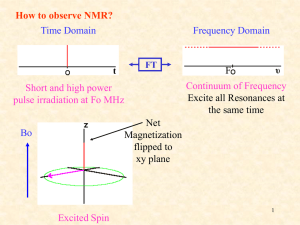
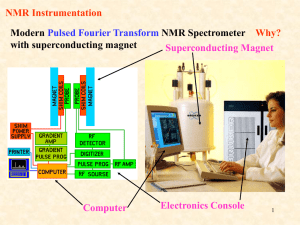
NMR Spectroscopy: 1H NMR Spectral Analysis
advertisement

Spring 2014 CHEM 212 – NMR Spectroscopy 1 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR 1. NMR is rarely used in a vacuum to do a “forensic” analysis of an unknown 2. NMR (all nuclei) is usually used: – To analyze the product of a chemical reaction along with IR – To elucidate the structures of natural products (like the spice lab compounds in CHEM 213) in conjunction with mass spectrometry (which gives molecular weights and formulas), IR and UV 3. In this course, you will be given one of three pieces of data with an 1H NMR for consideration: – A molecular formula – An IR spectrum – The first part of a chemical reaction – for example: O NaBH4 EtOH 2 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR Step 1: Do a quick assessment of the information you are given Molecular formula – one of the most important pieces of information Use the index of hydrogen deficiency (HDI) to determine the possible number of rings, double and triple bonds in the molecule: For a chemical formula: CxHyNzO (halogens count as Hs) HDI = x – ½ Y + ½ Z + 1 Example: C4H8O -- HDI = 4 – ½ (8) + ½ (0) + 1 HDI = 1 This compound contains either one double bond or one ring 3 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR Step 2: Do a quick assessment of the 1H NMR you are given – Is the molecule simple or complex? – Is the molecule aromatic, aliphatic or both? – What are the total number of resonances that you observe Be careful with overly simple spectra – remember a large molecule may appear to be small and simple if it is highly symmetrical Consider: Durene, C10H14 1H 4 NMR spectrum consists of two singlets! Spectral Analysis – 1H NMR NMR Spectral Analysis – Introductory 1H NMR Example: 1H NMR for C4H8 (which has an HDI of 1) 5 NMR Spectroscopy Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR Step 3: Use the integration along with the molecular formula to make sure you can find all of the 1Hs (this is 1H NMR after all!) If you do not have a molecular formula, use the integration to attempt to tabulate the number of 1Hs in the formula (does it make sense?) If one hydrogen appears to be “missing”, you may suspect it is acidic or exchangeable with the dueterated NMR solvent Remember, integration gives you the least common denominator of the total number of protons of each type Keep in mind organic molecules contain –C-H’s, -CH2- ’s, -CH3 ’s and multiples of chemically equivalent ones! 6 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis Continue our example: 43 For C4H8O we need to find 8 protons The integral for the quartet at d 2.5 measures 30 mm, the singlet at d 2.2 measures 44 mm, the triplet at d 1.1 measures 43 mm The ratio: 30:43:42 is roughly 2:3:3 2+3+3=8 We found all 8 protons 7 44 30 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR Step 4: Classify each of the proton resonances by using the general correlation table Reconsider the number of rings, double or triple bonds that are possible given the HDI, and reconcile this data with what the 1H chemical shifts are telling you Some hints: If you calculate an HDI > 4, you probably have an aromatic ring, and this should show on the spectrum If you calculate an HDI of 1 or 2 and see no protons that are part of an alkene and alkyne – suspect rings if no oxygen's are present, carbonyls if (C=O) they are 8 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis Continue our example: For C4H8O we have 3 families of chemically equivalent protons in a ratio of 2:3:3 or –CH2-, -CH3, -CH3 EWG CH3 Both the d 2.2 and 2.5 resonance correspond to protons on carbons next to electron withdrawing groups EWG The d 1.1 resonance corresponds to protons on carbons bound to other aliphatic carbons 9 R CH2 R CH3 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis Continue our example: We found –CH2-, -CH3 and –CH3, subtract these from our original formula: C4H8O - CH2 = C3H6O C3H6O – CH3 = C2H3O C2H3O – CH3 = CO We needed an HDI of 1 and there is no evidence for 1H-C=C on the 1H NMR, our missing HDI, and EWG is a C=O! 10 EWG CH3 EWG R CH2 R CH3 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR Step 5: Analyze the spin-spin coupling multiplets to elucidate the carbon chains of the molecule Hints: • Singlets indicate you have protons on carbons that have no chemically non-equivalent protons on any adjoining atom 11 • Multiplets mean you have chemically non-equivalent protons on adjoining carbons (or atoms), use the n+1 rule in reverse to find out how many • Spin-spin coupling or splitting is MUTUAL, if you observe a multiplet there must be another multiplet it is related to (split by) Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis For our example, this is trivial; we have concluded the C=O is the electron withdrawing group The –CH3 singlet at d 2.2 obviously has no 1Hs on adjoining carbons, as it is next to the carbonyl The d 2.5 –CH2- quartet is next to a –CH3 (n+1 = 4, so n = 3, it is next to a –CH3) The d 1.2 –CH3 is a triplet, so it is next to a –CH2 12 EWG CH3 EWG R CH2 R CH3 Spectral Analysis – 1H NMR NMR Spectroscopy NMR Spectral Analysis – Introductory 1H NMR Step 6: Construct the molecule and double-check consistency • Does the HDI match? Have you accounted for all atoms in the formula? • 13 From your constructed molecule, pretend you are trying to verify if that spectrum matches, and quickly re-do the problem Spectral Analysis – 1H NMR NMR Spectral Analysis We concluded we have: O H 2 H3C C C CH3 or 2-butanone C4H8O, HDI = 1 3 proton resonances 2 mutually coupled (split) 14 NMR Spectroscopy Spectral Analysis – 1H NMR Example 2: C9H9BrO HDI = 15 NMR Spectroscopy Spectral Analysis – 1H NMR Example 2: C9H9BrO HDI = 9 – ½ (10) + 0 + 1 = 5 16 NMR Spectroscopy Spectral Analysis – 1H NMR Example 2: C9H9BrO HDI = 9 – ½ (10) + 0 + 1 = 5 17 NMR Spectroscopy Spectral Analysis – 1H NMR Example 2: C9H9BrO HDI = 9 – ½ (10) + 0 + 1 = 5 18 NMR Spectroscopy O Br Spectral Analysis – 1H NMR Example 3: C4H10O HDI = 19 NMR Spectroscopy Spectral Analysis – 1H NMR Example 3: C4H10O HDI = 4 – ½ (10) + 0 + 1 = 0 20 NMR Spectroscopy Spectral Analysis – 1H NMR Example 3: C9H9BrO HDI = 0 21 NMR Spectroscopy Spectral Analysis – 1H NMR Example 3: C4H10O HDI = 0 22 NMR Spectroscopy HO Spectral Analysis – 1H NMR Example 4: C5H10O2 HDI = 23 NMR Spectroscopy Spectral Analysis – 1H NMR Example 4: C5H10O2 HDI = 5 – ½ (10) + 0 + 1 = 1 24 NMR Spectroscopy Spectral Analysis – 1H NMR Example 4: C5H10O2 HDI = 1 25 NMR Spectroscopy Spectral Analysis – 1H NMR NMR Spectroscopy Example 4: C5H10O2 O HDI = 1 26 HO Spectral Analysis – 1H NMR Example 5: C10H12O2 HDI = 27 NMR Spectroscopy Spectral Analysis – 1H NMR Example 5: C10H12O2 HDI = 10 – ½ (12) + 0 + 1 = 5 28 NMR Spectroscopy Spectral Analysis – 1H NMR Example 5: C10H12O2 HDI = 5 29 NMR Spectroscopy Spectral Analysis – 1H NMR Example 5: C10H12O2 HDI = 5 30 NMR Spectroscopy O OH Spectral Analysis – 1H NMR Example 6: C6H4ClNO2 HDI = 31 NMR Spectroscopy Spectral Analysis – 1H NMR Example 6: C6H4ClNO2 HDI = 6 – ½ (5) + ½ (1) + 1 = 5 32 NMR Spectroscopy Spectral Analysis – 1H NMR Example 6: C6H4ClNO2 HDI = 5 33 NMR Spectroscopy Spectral Analysis – 1H NMR Example 6: C6H4ClNO2 NMR Spectroscopy O O N HDI = 5 Cl 34