Document
advertisement

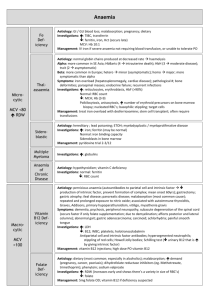
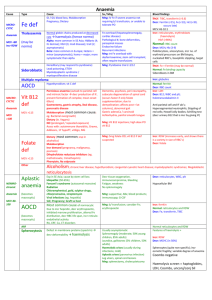
Patient Safety Preanalytical Phase Vladimir Palicka Charles University Hradec Kralove, Czech Republic International Symposium “Patient Safety”, Prague, April 12, 2013 Preanalytical Phase The Weakest Point in Quality Management International Symposium “Patient Safety”, Prague, April 12, 2013 The value of laboratory testing for diagnostics and therapy Quantitative at minimum 80-90 % of all objective data are results of laboratory or other complementary departments Qualitative high quality information only are of value, the others are dangerous To err is human: building a safer health system Kohn LT, Corrigan JM, Donaldson MS National Academy Press, Washington, DC, 2000 Errors in medicine 10-20 % of errors negatively influence health care quality > 3 % of errors are of direct influence on patient safety „the more tests, the more errors“ Laboratory error A defect occurring at any part of the laboratory cycle, from ordering tests to reporting results and appropriately interpreting and reacting to these ISO/PDTS 22367 negative/risky trends for quality Consolidation pre-analytical phase Decentralization (POCT) analytical quality Outsourcing pre- and post-analytical Downsizing, shortages total quality positive trends for quality Integration of automatization and informatics improved process control Standard Operation Procedures reduction of errors in all phases Improved contact with clinicians pre- and post-analytical phase Errors in laboratory medicine analytics approx 15 % (7-13%) preanalytics approx 62 % (46 – 68%) postanalytics approx 23 % (18 – 45%) Total Testing Process Improvement prevalence of errors was reduced by automation improved laboratory technology assay standardization informatics but mostly in analytical part ! Most common reasons of pre-analytical errors Haemolysis Misidentification Sampling error (wrong tube, inappropriate amount of the sample) Clotting Sample and/or request missing Wrong patient preparation Preanalytical errors Retrospective analysis 2001-2005 4.715.132 samples in 105 labs The most common reason for sample rejection Missing sample (37.5%) Haemolysis (29.3%) (serum 38.6%, plasma 68.4%) Alsina J: CCLM 2008, 46: 849 preanalytical errors misidentification wrong sampling pumping with fist wet skin tourniquet time sample mixing (inverting) time for transport and centrifugation Detection of inappropriateness Visual inspection of lipaemic, icteric and/or haemolysed samples is highly unreliable and should be replaced by automated systems (serum indices) Haemolysis upper „reference limit“ for free Hb plasma 20 mg/l serum 50 mg/l Visible haemolysis after centrifugation free Hb > 300 mg/l = 18.8 mmol/l (approximately 0.5% of Ery are lysed) Haemolysis - reasons in vivo – in vitro Up to 2% samples are haemolysed At minimum 50 possible reasons inherited-acquired haemolytic anaemia haemoglobinopathias HELLP syndrome drugs, infection artificial heart valves transfusion of incompatible blood Haemolysis – common reasons in vivo – in vitro Wet skin at sampling site Thin needle (usually < 21 G) Difficult venipucture Fragile veins Vacuum in tube is too high Wrong amount of blood for the amount of additive (anticoagulant) Haemolysis - reasons Inappropriate shaking the sample Temperature discomfort High centrifugation force Long centrifugation To early centrifugation Late serum/plasma separation Wrong separation barrier Re-centrifugation of gel-tubes Pneumatic sample transporting Haemolysis The most common reasons of the wrong samples Frequency 40 – 70% of all rejected samples (5-times more than any other reason) Haemolysis according dept Lippi G, CCLM 47: 616, 2009 Haemolysis increased concentration/activity: AST, ALT, CK, LDH, lipase creatinine, urea, Fe, Mg, P, K decreased concentration/activity: ALP, GGT Alb, bilirubin, Cl, G, Na Special care: newborn bilirubin !! Haemolysis Immunoassay False negative troponin T False increase of troponin I False increase of PSA Negative bias: testosterone, cortisol, FPIA Impossibility to measure: insulin, glukagon, CT, PTH, ACTH, gastrin In the case of haemolysis a) Correction of result(s) b) Release of results with flags and comments c) Information of ward and new-sample request In the case of haemolysis a) Result correction Methods with known interference (nm) rejected Release „unaffected“ results, only Potassium results corrected by recalculation Should we correct the results ? Haemolysis: potassium Linear correlation Should we use the „index“ or measured concentration ? different analyzers – different indexes different calculation of corrected K = K measured – (Hb mmol/l x 5.2) K measured– (Hb mmol/l x 10) Bland-Altman: uncertainty ± 0.4 mmol/l In the case of haemolysis a) Result correction Methods with known interference (nm) rejected Release „unaffected“ results, only Potassium results corrected by recalculation incorrect, error is too big ! intravascular haemolysis ? In the case of haemolysis b) Release of results with flags and comments Many types of comments Wrong decision is quite common Credibility of lab decreases Extreme situations? In the case of haemolysis c) Information of ward and new-sample request Nonconformity notification Laboratory book and hospital rules Quick reaction is necessary New sample request In the case of haemolytic sample Information to ward Consultation New sample request To err is human building a safer health system Kohn LT, Corrigan JM, Donaldson MS National Academy Press, Washington, DC, 2000 To err is human to delay is deadly Consumer Reports – Health Safe Patient Project.org Patient Identification Errors EQA - PAPA Australia, New Zealand 12-year period 59 participating laboratories 3.9 million specimens PAPA incident rate: 1.22 % most significant incident Patient or Sample Identification ! Quality System Requirements ISO 15189:2007 SOPs JCI: at least two patient identifiers Bracelets bar-codes RFID (radiofrequency identifier devices) automated systems The most common system Patient – Wards Wrist-bands, electronic order, bar-code sticks Laboratory Data terminal Hand-held bare code scanner Portable label printer software systems for patient identification barcodes Bar codes History: local grocery, 1948 Patent was applied for 1949 Patent issued 1952 Today: more that 2 dozen different linear bar code symbologies Most frequent used: Code 128, Code 39 Error rate expected 1:400.000 – 1.800.000 Most common sources of errors Printing defect in the barcode Suboptimal barcode orientation Lack of error detection Scanner resolution Sasavage N: Clin.Lab.News, 2011, Jan Errors in bar code technology More often in POCT More often on wristband than on paper Take care about printer heads Thick black line Turn the label stock by 90o Snyder ML, Clin.Chem. 2010, 56:1554 Sasavage N: Clin.Lab.News, 2011, Jan Sasavage N: Clin.Lab.News, 2011, Jan Sasavage N: Clin.Lab.News, 2011, Jan Errors in bar code technology More often in POCT More often on wristband than on paper Take care about printer heads Thick black line Turn the label stock by 90o Quality program Cleaning and bar code verifier use Snyder ML, Clin.Chem. 2010, 56:1554 systems for patient identification barcodes radio frequency identification (RFID) biometrics magnetic stripes optical character recognition „smart“ cards voice recognition causes of patient misidentification identical names China example 60 in-patient sampled in 32 of them (53 percent) common full name shared with 1 – 101 other patients attending the same hospital (Hong Kong) Lee AC: Int.J.Health Care Qual.Assur.Inc.Leadersh.Health Serv., 2005:18/1:15 Astion M: Clin.Lab.News 20110,Jan causes of patient misidentification identical names wristband „problems“ CAP: 2.6 % errors (missing wristband, ID, illegible, incorrect) wristband errors Join Commission on Accreditation CAP – Q-Probes mean wristband error rates 5.4 – 8.4 % after the introduction of QIM < 1.0 % wristband errors 4-years study 464 bed public hospital bar-coded wristbands total wristband error rates 10.6-16.5% training sessions total wristband error rates 0.4-1.5% Dhatt GS: CCLM 2001, 49/5: ?? wristband errors 2 hospitals in Sweden (230+152 beds) 295 nurses/phlebotomists questionnaire undesirable practice 9.6% not asking name and ID 17% not checking identity 79% not checking wristband during ID 43% using health care card for ID Wallin O: Scand.J.Caring Sci. 2010, 24/3: 581 Patient Identification Errors differences between type of labs transfusion medicine 0.05 % clinical chemistry up to 1 % most common reasons malpractice (low interest), low adherence to QSR high workflow wrong technique What about relabeling Very strict policy Blood and urine rarely will be candidates Sometimes indicated for irreplaceable specimens (cerebrospinal fluid, bone marrow, surgical) The risk of recollection is greater than a risk for relabeling… SOP Astion M: Clin.Lab.News 20110,Jan home mesage identification mistakes are not easily detectable no immediate harm or signal many steps – no personal responsibility mostly not systematic not considered as the big problem fear of blame human factor involved home mesage patient identification is common duty of clinicians, phlebotomists and clinical chemists technical equipment is necessary (but must be under the control) ISO, SOP, EQA are extremely important education and enthusiasm of people is the corner stone home mesage before any test we should be sure whom are we testing ! Patient safety and proper care is the target !