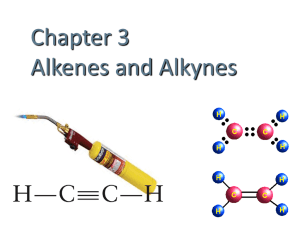
Unsaturated hydrocarbons

What are unsaturated hydrocarbons?
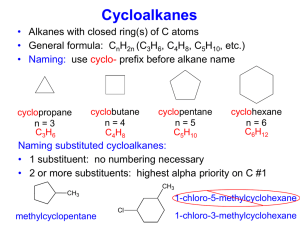
Saturated hydrocarbons
• maximum number of hydrogen atoms attached to each carbon atom.
• alkanes and cycloalkanes with single C-C bonds.
CH
3
—CH
2
—CH
3
Unsaturated hydrocarbons
• fewer hydrogen atoms attached to the carbon chain than alkanes.
• alkenes with double bonds.
• alkynes with triple bonds.
1
Aliphatic vs Aromatic
Hydrocarbons can be aliphatics
Straight chain
Alkanes, alkenes and alkynes
CH
3
—CH
2
—CH
3,
H
2
C=CH ─ CH
3 ,
HC ≡ C ─ CH
3
Hydrocarbons can be Aromatics
must have benzene ring
Let’s study aliphatic: alkenes?
The names of alkenes
• use corresponding alkane name.
• Change ending to – ene .
Alkene IUPAC Common
H
2
C=CH
2
H
2
C=CH ─ CH
3 eth ene prop ene ethyl ene propylene cyclohex ene
3
Ethene (Ethylene )
Ethene or ethylene
• alkene with the formula C
2
H
4
.
• two carbon atoms connected by a double bond.
• two H atoms bonded to each C atom.
• flat with all C and H atoms in same plane.
• used to accelerate ripening of fruits.
4
How do we name alkynes (aliphatic)?
The names of alkynes
• use corresponding alkane name.
• change ending to – yne .
Alkyne IUPAC Common
HC ≡ CH
HC ≡ C ─ CH
3 eth yne prop yne acetylene
5
Look for double or triple bonds carbon chain of an alkene or alkyne has four or more C atoms, number chain to give lowest number to first carbon in double or triple bond.
CH
2
=CH ─ CH
2
─ CH
3
1 2 3 4
CH
3
─ CH=CH ─ CH
3
1 2 3 4
CH
3
─ CH
2
─ C
C ─ CH
3
5 4 3 2 1
1-butene
2-butene
2-pentyne
6
Learning check
Write the IUPAC name for each of the following:
1. CH
2
=CH ─ CH
2
─ CH
3
2. CH
3
─ CH=CH ─ CH
3
CH
3
|
3. CH
3
─ CH=C ─ CH
3
4. CH
3
─ C
C ─ CH
3
7
Learning check
Write the IUPAC name for each of the following:
A. CH
3
─ CH
2
─ C ≡ C ─ CH
3
CH
3
B. CH
3
─ CH
2
─ C=CH ─ CH
3
8
What are cis and trans isomers?
In alkene double bond,
• is rigid.
• holds attached groups in fixed positions.
• makes cis/trans isomers possible.
CH
3
CH = CH cis
CH
3
CH
3
CH = CH trans CH
3
9
Possible cis-trans isomers !
Two isomers are possible when groups are attached to the double bond are different.
• cis isomer , groups are attached on same side of double bond.
• trans isomer , groups are attached on opposite sides.
Br Br
Br
C C C C
H H
H
H Br
H Br
H
C C
CH
3
(not cis or trans)
H
C C
Br
(not cis or trans)
H
Br cis1,2-dibromoethene trans1,2dibromoethene prefixes cis or trans are placed in front
Reactions of unsaturated hydrocarbons
In alkene and alkynes (aliphatic)
• double or triple bond easily broken,
• which makes double and triple bonds very reactive.
• reactants are added to carbon atoms in double or triple bond.
11
Let’s look at hydrogenation!
hydrogenation ,
• Hydrogen atoms add to carbon atoms of a double bond or triple bond.
• catalyst such as Pt or Ni used to speed up reaction.
• Adding H
2 to double bonds in vegetable oils
• Compounds with higher melting points.
• Solids at room temperature such as margarine, soft margarine, and shortening. some cis double bonds convert
• trans double bonds (more stable)
• cause change in fatty acid
• label states “partially” or “fully hydrogenated
H H
Pt
H
2
C CH
2
+ H
2
H
2
C CH
2
HC CH + 2H
2
Ni
H H
HC CH
H H 12
Let’s look at hydrogenation reaction
What are hydration reactions?
addition reaction called hydration,
• acid H + catalyst is required.
• water (HOH) adds to double bond.
• H atom bonds to one C in double bond.
• an OH bonds to other C.
CH
3
─ CH=CH ─ CH
3
+ H ─ OH
H +
H OH
│ │
CH
3
─ CH ─ CH ─ CH
3
14
More on hydration!
When hydration occurs with a double bond that has an unequal number of H atoms ,
• H atom bonds to C in double bond with more H.
• OH bonds to C in double bond with the fewer H atoms.
• Rich gets richer rule!
CH
3
─ CH=CH
2
+ H ─ OH
H +
OH H
│ │
CH
3
─ CH ─ CH
2
15
Another reaction of unsaturated hydrocarbons!
Polymers are
• large, long-chain molecules.
• found in nature, including cellulose in plants, starches in food, proteins, and DNA in body.
• also synthetic such as polyethylene and polystyrene,
Teflon, and nylon.
• have small repeating units called monomers .
• can be made from reaction of small alkenes.
16
Let’s look at polymerization closely!
In polymerization, small repeating units called monomers join to form a long chain polymer.
H
C C
H
+
H H
H
H
C C
H
H
+
H
C C
H
H H
Ethylene monomers monomer unit repeats chain continues
H H H H H H
C C C C C C
H H H H H H
Polyethylene n chain continues
17
What are aromatic Compounds?
Benzene is
• an aromatic compound .
• a ring of 6 C atoms and 6 H atoms.
• a flat ring structure drawn with three double bonds.
• represented by two structures because the electrons are shared among all the C atoms.
• has 6 electrons shared equally among the 6 C atoms.
• is also represented as a hexagon with a circle drawn inside.
18
Some aromatic compounds in nature and health
Vanillin
O
CH
Aspirin
O
COH O
C O CH
3
OCH
3
OH
Ibuprofen Acetaminophen
O
NH C CH
3
OH
H
3
C
CH
3
CH CH
2
CH
3
CH
O
COH
19
Naming aromatic compounds
Aromatic compounds are named
• with benzene as parent chain.
• with one side group named in front of benzene .
CH
3 Cl methyl benzene chloro benzene
20
Some common names
Some substituted benzene rings
• have common names that have been in use for many years.
• with a single substituent use a common name or are named as a benzene derivative.
CH
3
NH
2
OH toluene aniline phenol
(methylbenzene) (benzenamine) (hydroxybenzene)
21
Naming aromatic compounds
When two groups are attached to benzene ring, the ring is numbered to give the lowest numbers to the side groups.
CH
3
Cl OH
Cl
Cl
Cl
3-chlorotoluene 1,4-dichlorobenzene 2-chlorophenol
22