GAS TRANSPORT & CONTROL OF RESPIRATION
advertisement

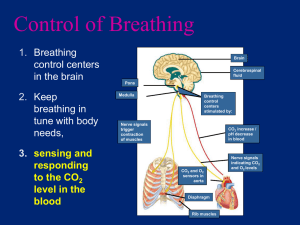
GAS TRANSPORT & CONTROL OF RESPIRATION GAS TRANSPORT • Blood transports Oxygen and Carbon dioxide between lungs and the tissues of the body • These gases are transported in different states 1. Dissolved in plasma 2. Chemically combined with hemoglobin 3. Converted to a different molecule Oxygen Transport • Due to low solubility, only 1.5 % of oxygen is dissolved in plasma • 98.5 % of oxygen combines with hemoglobin • Each Hb consists of a globin portion composed of 4 polypeptide chains • Each Hb also contains 4 iron containing pigments called heme groups • Up to 4 molecules of O2 can bind one Hb molecule because each iron atom can bind one oxygen molecule • There are about 250 million Hb hemoglobin molecules in one Red Blood Cell • When 4 oxygen molecules are bound to Hb, it is 100% saturated, with fewer, it is partially saturated • Oxygen binding occurs in response to high partial pressure of Oxygen in the lungs • Oxygen + Hb Oxyhemoglobin (Reversible) • Cooperative binding Hb’s affinity for O2 increases as its saturation increases (similarly its affinity decreases when saturation decreases) • In the lungs where the partial pressure of oxygen is high, the rxn proceeds to the right forming Oxyhemoglobin • In the tissues where the partial pressure of oxygen is low, the rxn reverses. OxyHb will release oxygen, forming again Hb (or properly said deoxyhemoglobin) Oxygen-Hemoglobin Dissociation Curve • Hb saturation is determined by the partial pressure of Oxygen • @ High partial pressures of O2 – lungs – Hb is 98% saturated • @ Low partial pressures of Oxygen – tissues – Hb is only 75% saturated • “S” shape is a trademark of its cooperative binding interaction – the binding of one oxygen molecule increases Hb’s affinity for binding additional oxygen molecules Other factors altering Hb saturation • Low pH (Carbonic Acid, Lactic Acid) • High Temperature • High 2,3 DiphosphoGlycerate concentration (DPG) • High partial pressure of Carbon Dioxide • These conditions decrease Hb’s affinity for oxygen, releasing more oxygen to active cells • Example: Vigorous physical exercise • Contracting muscles produce metabolic acids such as lactic acid which lower the pH, more heat and more carbon dioxide. • In addition 2,3 DPGA is produced during conditions of higher temperature and lower partial pressures of oxygen Acting together or individually, these conditions lead to a decrease in Hemoglobin’s activity for Oxygen, releasing more Oxygen to the tissues (muscles) BOHR EFFECT Bohr Effect • Bohr Effect refers to the changes in the affinity of Hemoglobin for oxygen • It is represented by shifts in the Hb-O2 dissociation curve • Three curves are shown with progressively decreasing oxygen affinity indicated by increasing P(50) • • • • 1. 2. 3. 4. • SHIFT to the RIGHT Decreased affinity of Hb for Oxygen Increased delivery of Oxygen to tissues It is brought about by Increased partial pressure of Carbon Dioxide Lower pH (high [H+]) Increased temperature Increased levels of 2,3 DPGA Ex: increased physical activity, high body temperature (hot weather as well), tissue hypoxia (lack of O2 in tissues) • • • • 1. 2. 3. 4. • SHIFT to the LEFT Increased affinity of Hb for Oxygen Decreased delivery of Oxygen to tissues It is brought about by Decreased partial pressure of Carbon Dioxide Higher pH (low [H+]) Decreased temperature Decreased levels of 2,3 DPGA Ex: decreased physical activity, low body temperature (cold weather as well), satisfactory tissue oxygenation Carbon Dioxide Transport • Produced by cells thru-out the body • CO2 diffuses from tissue cells and into the capillaries • 7% dissolves in plasma • 93% diffuses into the Red Blood Cells • Within the RBC ~23% combines with Hb (to form carbamino hemoglobin) and ~ 70% is converted to Bicarbonate Ions which are then transported in the plasma • In the lungs, which have low Carbon Dioxide partial pressure, CO2 dissociates from CarbaminoHemoglobin, diffuses back into lungs and is exhaled • Within the RBC, CO2 combines with water and in the presence of carbonic anhydrase it transforms into Carbonic acid • Carbonic acid then dissociate into H+ and HCO3• In the lungs CO2 diffuses out into the alveoli. This lowers the partial press. Of Co2 in blood, causing the chemical reactions to reverse • Other gases have different affinities for hemoglobin • CO carbon monoxide has more than 250 times the affinity for Hb than oxygen. It will quickly and almost irreversibly bind to Hb CO poisoning • NO nitrogen oxide has more than 200,000 times the affinity for Hb than oxygen. Irreversible bind • CO and O2 bind to same site on Hb • CO2 and O2 bind to different sites on Hb • Myoglobin (in muscle cells) binds more tightly to oxygen than Hb but NOT cooperatively (Mb serves as temporary intracellular O2 storage mechanism useful in muscle contraction) Llama and Vicuna • Llama & Vicuna live in the Andes Mts. South America • Oxygen dissociation curves are located to the left of other mammals • Higher oxygen affinity of the blood of these animals aids in oxygen uptake at the low pressure of high altitude High Altitude Adaptations for us… • Chronic Mountain Sickness (ventilatory depression, polycythemia, heart failure) R.I.P. • At high altitude initially the person is hyperventilating • After some time however… • Hb/RBC production increases (more oxygen carrying capacity) • 2,3 DPGA concentration rises in RBCs shifting the curve to the right, improving O2 tissue delivery • Increased sensitivity to concentrations of [H+], CO2, pH and their respective variations That’s exactly why sportsmen (real football players for instancewrongfully called “soccer players” here) train in the mountains To improve physical performance ! • At similar pH and Co2, small mammals have lower oxygen affinity. Improved delivery of oxygen in the tissues to sustain the high metabolic rate of a small animal • Higher oxygen affinity of the fetal blood helps in the transfer of oxygen to the fetal blood in the placenta • Fetus – higher affinity- shift left • Fetus [Hb]~200g/L • Mother [Hb]~135g/L • Normal [Hb]~150g/L Control of Respiration • Basic rhythm is controlled by respiratory centers located in medulla and brainstem • An inspiratory center sends impulses via nerves to the effectors: diaphragm and intercostals muscles • Normal breathing rate @ rest is about 12 to 15 breaths a minute • Chemo receptors located thru out the body modify the breathing rhythm by responding to changes in partial pressures of Co2, O2, pH. • Central chemoreceptors medulla changes in pH • Peripheral chemoreceptors: carotid body, aortic bodies monitor values of arterial blood: Pco2, Po2, pH Carbon dioxide is the most important factor controlling depth and rate of breathing For all other inquiries, please refer back to the BOHR Effect. • HYPERVENTILATION • Increased rate and depth of breathing if • Low pp. O2 • High pp. CO2 • Low pH, High [H+] • High Temperature • High 2,3 DPGA • High metabolic requirements • Shift to the RIGHT • HYPOVENTILATION • Decreased rate and depth of breathing if • High pp O2 • Low pp CO2 • High pH, Low [H+] • Low Temperature • Low 2,3 DPGA • Low metabolic requirements • Shift to the LEFT Other factors that affect respiration • Pain & strong emotion • Pulmonary Irritants (dust, smoke, noxious fumes, excess mucus) • Voluntary control (ALWAYS OVERIDDEN) • Lung Hyperinflation (stretch receptors in pleurae send inhibitory signals protecting against hyperinflation) • Exercise and ventilation