Electrolytic Cells
advertisement
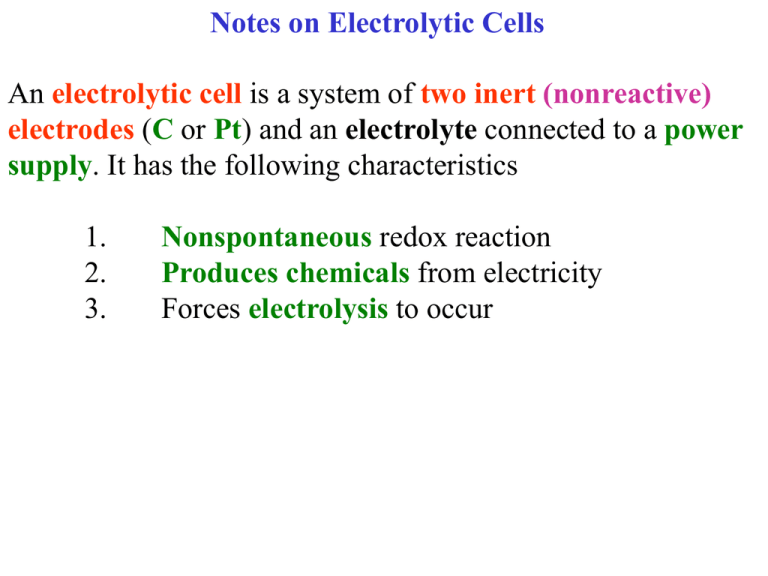
Notes on Electrolytic Cells An electrolytic cell is a system of two inert (nonreactive) electrodes (C or Pt) and an electrolyte connected to a power supply. It has the following characteristics 1. 2. 3. Nonspontaneous redox reaction Produces chemicals from electricity Forces electrolysis to occur When analyzing an electrolytic cell, your first and most important step is to determine the oxidation and reduction reactions. Electrolytic Cell Main Rule The electrode that is connected to the -ve terminal of the power supply will gain electrons and therefore be the site of reduction. Other Rules: For Electrochemical and Electrolytic Cells Oxidation always occurs at the anode and reduction at the cathode Electrons flow through the wire and go from anode to cathode Anions (- ions) migrate to the anode and cations (+ions) migrate towards the cathode. 1. Draw and completely analyze a molten NaBr electrolytic cell. 1. Draw and completely analyze a molten NaBr electrolytic cell. Draw a beaker, two inert electrodes wired to a power supply. 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Draw a beaker, two inert electrodes wired to a power supply. 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Label the electrode with Pt or C. 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Pt Label the electrode with Pt or C. Pt 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Pt Add the electrolyte Pt 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Add the electrolyte Molten or liquid means no water! Pt Pt Na+ Br- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Label the negative and positive electrodes Pt Pt Na+ Br- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Supply DC + Label the negative and positive electrodes Pt Pt _ + Na+ Br- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + The negative is reduction and the positive is oxidation. Pt Pt _ + Na+ Br- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction The negative is reduction and the positive is oxidation. Na+ Br- + oxidation 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode The anode is oxidation and the cathode is reduction. Na+ Br- + oxidation anode 1. Draw and completely analyze a molten NaBr electrolytic cell. The anion Power Source migrates to the + anode and the cation to the cathode. Pt Pt _ reduction cathode Na+ Br- + oxidation anode 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode The anode reaction is the oxidation of the anion. Na+ Br- + oxidation anode 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode The anode reaction is the oxidation of the anion. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode The Cathode reaction is the reduction of the cation. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) The Cathode reaction is the reduction of the cation. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) Gas Br2 is produced at the anode. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e- 1. Draw and completely analyze a molten NaBr electrolytic cell. Power Source + Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) Liquid Na is produced at the cathode. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e- 1. Draw and completely analyze a molten NaBr electrolytic cell. The potential for Power Source each half reaction + is calculated and the oxidation sign is reversed Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e- 1. Draw and completely analyze a molten NaBr electrolytic cell. The potential for Power Source each half reaction + is listed and the oxidation sign is reversed Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v 1. Draw and completely analyze a molten NaBr electrolytic cell. The overall redox Power Source reaction is + written. Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v 1. Draw and completely analyze a molten NaBr electrolytic cell. The overall redox Power Source reaction is + written. Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v Na+ Br- 2Na+ + 2Br- → Br2(g)+ 2Na(l) + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v E0 = -3.80 v 1. Draw and completely analyze a molten NaBr The minimum theoretical electrolytic cell. Power Source + voltage MTV required to force this nonspontaneous reaction to occur is the negative of the cell potential. Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v Na+ Br- 2Na+ + 2Br- → Br2(g)+ 2Na(s) + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v E0 = -3.80 v 1. Draw and completely analyze a molten NaBr The minimum theoretical electrolytic cell. Power Source + Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v 2Na+ + 2Br- → Br2(g)+ 2Na(s) voltage MTV required to force this nonspontaneous reaction to occur is the negative of the cell potential. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v E0 = -3.80 v MTV = +3.80 v 1. Draw and completely analyze a molten NaBr Electrons flow through electrolytic cell. Power Source + Pt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v 2Na+ + 2Br- → Br2(g)+ 2Na(s) the wire from anode to cathode. Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v E0 = -3.80 v MTV = +3.80 v 1. Draw and completely analyze a molten NaBr Electrons flow through electrolytic cell. e- the wire from anode to cathode. Power Source + ePt Pt _ reduction cathode 2Na+ + 2e- → 2Na(l) -2.71 v 2Na+ + 2Br- → Br2(g)+ 2Na(s) Na+ Br- + oxidation anode 2Br- → Br2(g)+ 2e-1.09 v E0 = -3.80 v MTV = +3.80 v 2. Draw and completely analyze a 1.0 M KI electrolytic cell. 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Add the ions. (aq) or M or solution means water. Pt Pt K+ IH2O 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Label the -, +, anode, cathode, oxidation, and reduction. Pt Pt K+ IH2O 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode reduction Label the -, +, anode, cathode, oxidation, and reduction. K+ IH2O + Anode oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode reduction The cation and water migrate to the cathode K+ IH2O + Anode oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode reduction The cation and water migrate to the cathode K+ IH2O + Anode oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode reduction The cation or water reduces. The higher one on the chart is most spontaneous and occurs. K+ IH2O + Anode oxidation Cl2 + 1/2O2 + 2e- → 2Cl- 2H+(10-7M) + 2e- 2H2O + 2e- 1.36 v → H20 → 2H2 + 2OH- -0.42 v Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → K(s) -2.93 v 0.82 v Cl2 + 1/2O2 + 2e- → 2Cl- 1.36 v 2H+(10-7M) → H20 0.32 v Reduction of water 2H2O + 2e- → 2H2 + 2OH- -0.42 v Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → K(s) -2.93 v Cl2 + 2e- → 2Cl- 1/2O2 + 2H+(10-7M) → H20 0.32 v Oxidation of water 1.36 v Reduction of water 2H2O + 2e- → 2H2 + 2OH- -0.42 v Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → K(s) -2.93 v Cl2 + 2e- → 2Cl- 1/2O2 + 2H+(10-7M) → H20 0.32 v Oxidation of water 1.36 v Reduction of water 2H2O + 2e- Zn2+ Reduction of K+ K+ + → 2H2 + 2OH- -0.42 v → Zn(s) -0.76 v + 2e- 1e- → K(s) -2.93 v Cl2 + → 2Cl- 2e- 1.36 v → H20 0.32 v Oxidation of water 1/2O2 + 2H+(10-7M) strongest oxidizing agent or highest select most spontaneous reaction Reduction of water → 2H2(g) + 2OH- -0.42 v 2H2O + 2e- Overpotential Effect- treat water as if it were just below Zn Zn2+ Reduction of K K+ + 2e- → Zn(s) -0.76 v + 1e- → K(s) -2.93 v The overpotential effect is a higher than normal voltage required for the half reaction. This is often due to extra voltage required to produce a gas bubble in solution. 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction The cation or water reduces. The higher one on the chart is most spontaneous and occurs. K+ IH2O + Anode oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction 2H2O+2e- → 2H2+ 2OH-0.41 v The cation or water reduces. The higher one on the chart is most spontaneous and occurs. K+ IH2O + Anode oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction 2H2O+2e- → 2H2+ 2OH-0.41 v The anion + water goes to the anode. K+ IH2O + Anode oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction 2H2O+2e- → 2H2+ 2OH-0.41 v For oxidation the most spontaneous reaction is found on the redox chart and is lowest. K+ IH2O + Anode oxidation → 2Cl- Cl2 + 2e- 1.36 v 1/2O2 + 2H+(10-7M) + 2e- → H20 0.82 v Oxidation of water I2(s) → + 2e- 2I- 0.54 v → 2H2 + 2OH- -0.42 v Reduction of water 2H2O + 2e- Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → K(s) -2.93 v → 2Cl- Cl2 + 2e- 1/2O2(g) + 2H+(10-7M) → I2(s) → + 2e- 1.36 v H20 0.82 v Oxidation of water 2I0.54 v Oxidation of I- Reduction of water 2H2O + 2e- → 2H2 + 2OH- -0.42 v Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → K(s) -2.93 v → 2Cl1.36 v overpotential effect means water is here Cl2 + 2e- 1/2O2 + 2H+(10-7M) → H20 0.82 v Oxidation of water → 2I0.54 v Oxidation of I- I2(s) + 2e- Reduction of water 2H2O + 2e- → 2H2 + 2OH- -0.42 v Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → -2.93 v K(s) → 2Cl1.36 v overpotential effect means water is here Cl2 + 2e- 1/2O2 + 2H+(10-7M) → I2(s) H20 0.82 v Oxidation of water → + 2e- 2I0.54 v Oxidation of Ipick strongest reducing agent- lower Reduction of water 2H2O + 2e- → 2H2 + 2OH- -0.42 v Zn2+ + 2e- → Zn(s) -0.76 v K+ + 1e- → -2.93 v K(s) 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction 2H2O+2e- → 2H2+ 2OH-0.41 v For oxidation the most spontaneous reaction is found on the redox chart and is lowest. K+ IH2O + Anode Oxidation 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction 2H2O+2e- → 2H2+ 2OH-0.41 v For oxidation the most spontaneous reaction is found on the redox chart and is lowest. K+ IH2O + Anode Oxidation 2I- → I2(s) + 2e-0.54 v 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Pt Pt Cathode Reduction 2H2O +2e- → 2H2+ 2OH-0.41 v Write the overall reaction with the cell potential. K+ IH2O + Anode Oxidation 2I- → I2(s) + 2e-0.54 v 2. Draw and completely analyze a 1.0 M KI electrolytic cell. Power Source + Write the overall reaction with the cell potential. Pt Pt Cathode Reduction 2H2O+2e- → 2H2+ 2OH-0.41 v K+ IH2O 2H2O+ 2I- → 2H2+ I2(s) + 2OH- + Anode Oxidation 2I- → I2(s) + 2e-0.54 v E0 = -0.95 v 2. Draw and completely analyze a 1.0 M KI electrolytic cell. e- Power Source + Write the overall reaction with the cell potential. ePt Pt Cathode Reduction 2H2O+2e- → H2+ 2OH-0.41 v + Anode Oxidation K+ IH2O 2H2O+ 2I- → H2+ I2(s) + 2OH- 2I- → I2(s) + 2e-0.54 v E0 = -0.95 v MTV = +0.95v







![Redox_equations[1].](http://s2.studylib.net/store/data/005611618_1-b55ee2e3a52621e48ab97cc179174553-300x300.png)
