Chemistry Note PowerPoint
advertisement

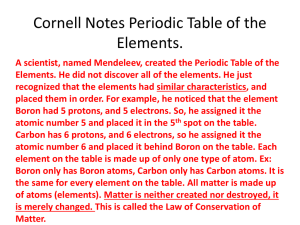
th 7 Grade Chemistry Elements and Atoms What is Chemistry? • Chemistry is the study of the properties of matter and how matter changes What is Matter? • Matter is anything that has mass and takes up space What are some examples of objects that contain matter? What is an Element? • An element is a substance that cannot be broken down into any other substance by chemical or physical means What does that mean? • Elements are the simplest substances • What are some examples of elements? What is an Element? What are some examples of elements? • Oxygen • Hydrogen • Gold • Silver ……..just to name a few How many elements are there? • There are 118 known elements on the Earth. What does that mean? • Everything on the Earth is made of one or more of those 118 elements • Elements of the periodic table song http://www.youtube.com/watch?v=zUDDiWtFtEM What is a compound? • A Compound is a pure substance made of two or more elements What are some examples of compounds? • Water • Carbon dioxide • Salt • Table sugar What is a compound? • A compound and molecule are represented by a chemical formula which shows the element in the compound and the ratio. What is a chemical bond? • A chemical bond is the force of attraction between two atoms, when they combine. • Atoms combine to form larger particles called molecules • A molecule is simply two or more atoms held together by a chemical bond • Oxygen molecule • http://www.brainpop.com/science/matterandchemistry/chemicalbonds/preview.weml What happens when two different atoms combine? When elements are chemically combined, they form compounds having properties that are different from those of the uncombined elements Example – salt (NaCl) Sodium Properties Chlorine Properties • Silver in color • Green • Solid • Gas • Metal • Low density • Very reactive • Poisonous • Explodes when comes into contact with water. • Very reactive Properties of Salt • Solid • White in color • Not reactive • Not poisonous • Does not explode when coming into contact with water • Soluble What is a mixture? • A mixture is made of two or more substances—element, compound, or both– that are in the same place but ARE NOT chemically combined. What is a homogeneous mixture? • A homogeneous mixture is a mixture in which the parts are so evenly mixed you can not tell the difference between them. • A solution is an example of a homogeneous mixture. It is formed when one substance is dissolved into another. What is a heterogeneous mixture? • In a heterogeneous mixture you can see the different parts How is a mixture different from a compound? • Each substance in a mixture keeps its individual properties. Also the parts are not combined in a set ratio. Atoms, Bonding, and the Periodic Table What is an atom? • An atom is the basic particle from which all elements are made • Atoms make up all the elements • Are the smallest pieces that retain physical and chemical properties • “building blocks” of matter • http://www.youtube.com/watch?v=cuXxSQEYDR8 What are subatomic particles? • An atom consists of a nucleus surrounded by one or more electrons. • Neutron found in the nucleus of an atom (has no charge) • Protons found in the nucleus, have a positive electric charge (+) • Electrons move rapidly around the nucleus and have a negative charge (e-) What is a cloud of electrons?? • An atom has a positive center known as the nucleus surrounded by a “cloud” of negative charge. What is a cloud of electron? • Most of the atom’s volume is the space in which electrons move. • The space is huge compared to the amount of space taken by the nucleus. • It symbolizes where electrons are LIKELY to be. • An electrons movement is related to its energy level, or the specific amount of energy that it has. • http://www.brainpop.com/science/matterandchemistry/atomicmodel/ Comparing Particle Size Valance Electrons and Bonding • An atom’s valance electrons are those that have the highest energy levels and are held most loosely. • The number of valance electrons determine many properties of that element, including the ways in which the atom combines with other atoms What is the periodic table? • The periodic table is a system used worldwide for organizing elements into categories • http://www.brainpop.com/science/matterandchemistry/periodictableofelement s/ Organizing the Elements • Each square on the periodic table is representing one of the known elements that make up all matter on Earth. • Each element is represented by a symbol usually consisting of one or two letters • Look at figure 10 on page 14 Organizing the elements • The atomic number of an element is the number of protons in the nucleus of an atom. • As you look at the elements from left to right on the periodic table, what do you notice about the atomic number of the elements? Finding Data on Elements Finding Data on the periodic table • In order to find the out how many subatomic particles an atom of a particular element has YOU MUST USE THE PERIODIC TABLE. • The periodic table purpose is to help you understand properties of the elements without having to memorize them for 118 elements HELPFUL HINTS TO DETERMINE THE NUMBER OF SUBATOMIC PARTICLES • The atomic number of an ATOM = the number of electrons • The atomic number of an ATOM = the number of protons • The atomic weight – atomic number = the number of neutrons Organization of the Periodic Table • Remember the periodic table is arranged by atomic number. • Atomic numbers as they increase from left to right, and read across each row. • The Properties of an element can be predicated from its location in the periodic table. Periods Periods • The table is arranged in horizontal rows called periods • A period contains different elements that have different properties • As you move from left to right in a period the properties change in pattern Groups Groups • The modern period periodic table has 7 periods which form 18 vertical columns. • The elements in a columns are called a group • The groups are number from 1-18 going from left to right. Group • Most groups are named for the first element in the group. • Elements in each group have similar properties and react in similar ways. How the periodic table works • The periodic table is based on the structure of atoms, especially the arrangement of the electrons. • As the number of protons – or atomic number– increases, the number of electrons also increase. As a result, the properties of the element change in a regular way across a period. • What do you notice about figure 11? How the periodic table works • Except for period 1, a period ends when the number of valance electrons reaches eight • THIS REPEATING PATTERN MEANS THAT ELEMENTS WITHIN THE SAME GROUP ALWAYS HAVE THE SAME NUMBER OF VALANCE ELECTRONS. Electron Dot Diagram • Each element has a specific number of valance electrons ranging from 1-8 • An electron dot diagram is a way to show the number of electrons in the element. Bohr’s Diagram • To display the entire atom you will use Bohr’s diagram • Each circle represent an energy level. • The farther away from the nucleus the more energy the electron has Chemical formulas and names • Compounds can be represented by chemical formulas • A chemical formula is a combination of symbols that shows the ratio of elements in a compound. Chemical formulas and names • A subscript tells you the ratio of the elements in the compound. • For every one hydrogen there are two oxygen atoms Observing Chemical Change What are the two types of properties? • Physical properties • chemical properties What is a physical property? • Physical property - is a characteristic of a pure substance that can be observed without changing it into another substance Types of physical properties • density • melting point • boiling point • solubility • texture • color • odor What are the two types of properties? • What is a chemical property? • Chemical property – is a characteristic of a pure substance that describes its ability to change into a different substance Types of chemical property • Ability to react • Flammability • Corrosiveness • Tarnishing What is the difference between a physical and a chemical change? http://www.brainpop.com/science/matterandchemistry/propertychan ges/ What is a physical change? • A physical change is any change that alters the appearance of matter but does not any change any substance into another substance. • In other words, a physical change only changes what an object looks like. • What some examples of a physical change? Physical Change • A substance that undergoes a physical change is still the same substance after the change • The substance still has the same properties • A physical change has occurred when: a. A change in the state of matter has occurred b. A change in the shape or form occurs What is a chemical change? • A change in matter that produces one or more new substances is called a chemical change or chemical reaction • Unlike physical change, a chemical change produces substance with properties that are different from the original substance. • What are some examples of a chemical change? Examples of a chemical change Chemical changes • Chemical changes occur when bonds break and new ones form. • As a result the new substances that are produced will have different physical and chemical properties than the original substances. Evidence for chemical reactions • Chemical reactions involve two main kinds of changes that you can observe – formation of new substances and a change in energy. Formation of a new substance = new properties • Changes in properties result when a new substance has been formed. • Things to look for: • Color change • A solid may appear when two liquids are combined, also known as a precipitate. • Gas is produced from combined solids or liquids • Formation of bubbles How do you describe a chemical reaction? A chemical equation • A chemical equation is a short easy way to show a chemical reaction using symbols instead of words. • Chemical equations use chemical formulas and other symbols instead of words to summarize a reaction • http://www.brainpop.com/science/matterandchemistry/chemicalequations/preview.we ml Formulas in equations • A chemical formula is a combination of symbols that represents the elements in a compound. • The formula for carbon dioxide is above. This tells us that in every molecule there are two oxygen atoms and one carbon atom Structure of an Equation • All chemical equations have a common structure • Every equation tells what substances that you start with and the substance that you end with in a chemical reaction. • Reactants = substances that you start with at the beginning of a reaction • Products = substances that you end with after a reaction has occurred • Subscript = the number in a chemical formula that tells the number of atoms in a molecule • Coefficient = is the number placed in front of a chemical formula in an equation, it tells you how many atoms or molecules there are Changes in energy • A change in energy occurs during a chemical reaction • As matter changes it can either absorb or release energy. • An endothermic reaction is a reaction in which energy is absorbed • TEMPERATURE DECREASES!!! Changes in energy • An exothermic reaction is a reaction that release energy • TEMPERATURE INCREASES What is conservation of mass? • The fact that matter is not created nor destroyed in any chemical or physical change is called The law of conservation of mass • No mass is lost, because during a chemical change, atoms are not lost or gained, only rearranged • http://www.brainpop.com/science/matterandchemistry/conservationofmass/