File - Miss Dickinson`s Chemistry
advertisement

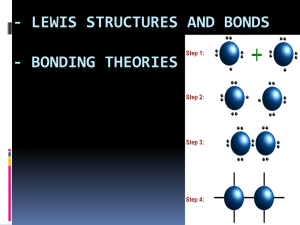
Shapes and Polarity Vocabulary • VSEPR model • Linear • Trigonal planar • Tetrahedral • Trigonal pyramidal • Bent • Trigonal bipyramidal • Octahedral • Polarity • Non-polar covalent bond • • • • • • • • • Polar covalent bond Ionic bond Symmetrical Asymmetrical Intermolecular Interaction Van der Waals Force London Dispersion Force Dipole-Dipole Hydrogen Bonding Objectives 1. The VSEPR model is used to determine molecular shape. 2. Describe how electronegativity is used to determine bond type. 3. Compare polar and nonpolar covalent bonds and polar/nonpolar molecules. 4. Define the interactions between molecules. 5. Draw and apply the intermolecular forces between molecules. VSEPR Model • The shape of a molecule determines many of its physical and chemical properties. • Molecular geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion model, or VSEPR model which minimizes the repulsion of shared and unshared atoms around the central atom. VSEPR Model (cont.) • Electron pairs repel each other and cause molecules to be in fixed positions relative to each other. • Unshared electron pairs also determine the shape of a molecule. • Electron pairs are located in a molecule as far apart as they can be. VSEPR Model (cont.) VSEPR Model (cont.) VSEPR Model (cont.) Section 8.4 Assessment The two lone pairs of electrons on a water molecule do what to the bond angle between the hydrogen atoms and the oxygen atom? A. They attract the hydrogen atoms and increase the angle greater than 109.5°. A 0% 0% A B C D 0% 0% D D. They create resonance structures with more than one correct angle. C C. They do no affect the bond angle. A. B. C. D. B B. They occupy more space and squeeze the hydrogen atoms closer together. Section 8.4 Assessment The sp3 hybrid orbital in CH4 has what shape? A. linear B. trigonal planar D A 0% C D. octahedral A. A B. B C. C 0% 0% 0% D. D B C. tetrahedral Electronegativity and Bond Character Polarity: Having poles (Having opposite ends) e.g. magnets have a North and South pole There are 2 ways to share electrons but they can also be transferred -“non-polar” covalent bonds: equal sharing of the e- pair -polar covalent bonds: unequal sharing of the e- pair - ionic bonds: transfer of e-’s from a metal to a nonmetal Electronegativity and Bond Character • Noble gases are not listed with electronegativity because they generally do not form compounds. Electronegativity and Bond Character Bond type is based on the electronegativity difference between the two bonded atoms. 0 to 0.4 = nonpolar covalent bond 0.5 to 2.0 = polar covalent bond Above 2.0 = ionic bond Practice Problems: Determine the type of bond that forms between the atoms in the following compounds. CO2 NaCl CH4 Electronegativity and Bond Character • Unequal sharing of electrons results in a polar covalent bond. • Polar covalent bonds form when atoms pull on electrons in a molecule unequally. • Electrons spend more time around one atom than another resulting in partial charges at the ends of the bond called a dipole. Polar Covalent Bonds (cont.) • Covalently bonded molecules are either polar or non-polar. • Non-polar molecules are not attracted by an electric field. • Polar molecules align with an electric field. Polarity of Molecules A polar molecule is like a magnet; one side is slightly positive and the other side is slightly negative. (Polar molecules are also known to have dipole moments.) Polarity depends on the shape and symmetry of the molecule. -symmetrical molecules = nonpolar - asymmetrical molecules = polar Polar molecules are moved by static charges. Polar Covalent Bonds (cont.) • Compare water and CCl4. • Both bonds are polar, but only water is a polar molecule because of the shape of the molecule. Properties of Covalent Molecules Covalent Molecules: - Insulators of electricity - formed between two nonmetal - usually have low melting points - solubility in water varies: (polar =dissolve; nonpolar = insoluble) -forms covalent cystalline solids. For a compound to to conduct electricity it has: 1. Charged Particles (ion) 2. Particles Free to Move (liquid or aqueous phase) The Strength of Covalent Bonds • The strength depends on the distance between the two nuclei, or bond length. • As length increases, strength decreases. The Strength of Covalent Bonds (cont.) • The amount of energy required to break a bond is called the bond dissociation energy. • The shorter the bond length, the greater the energy required to break it. Properties of Ionic Compounds Ionic compouds… -conduct of electricity when dissolved water or are melted. -formed between metal and nonmetals -have high melting points -usually very soluble in water -form ionic crystalline solids (lattice) INTERmolecular Attractions In order for molecules to stick together, they must be attracted to one another. The weak attractions between one molecule and another are called Van der Waals forces. They cause gas particles to stick together and condense at low temperatures. London Dispersion Forces (LDF’s) There are three types of intermolecular forces: 1. London Dispersion forces: – caused by random electron motion – generally stronger with more electrons in the molecule − exist between all types of molecules − This force causes Br2 to be a liquid and I2 to be a solid at room temperature. Dipole Interaction Forces 3. Dipole-Dipole interactions: – caused by the attraction of the positive side of one polar molecule and the negative side of a different polar molecule Hydrogen Bonds Hydrogen Bonding is a special type of dipole interaction. This attraction between molecules is not a chemical bond. Electrons are not being shared or transferred but the interaction is SUPER STRONG (strong forces between molecules) Happens between H and N, O or F Intermolecular Forces Substances that contain stong intermolecular forces have a higher melting point and a higher boiling point. Liquids containing strong intermolecular forces have higher surface tension and a higher viscosity. Surface Tension Section 8.5 Assessment The force between water molecules is what kind of intermolecular force? A. induced dipole B. hydrogen bond D A 0% C D. partial dipole A. A B. B C. C 0% 0% 0% D. D B C. sigma bond Section 8.5 Assessment What kind of bond occurs within a molecule with unequal sharing of electron pairs? A. ionic bond A 0% D D. polar covalent bond C C. non-polar covalent bond A. A B. B C. C 0% 0% 0% D. D B B. sigma bond