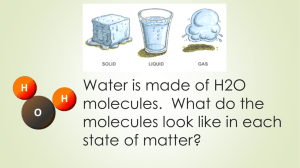
gas
advertisement
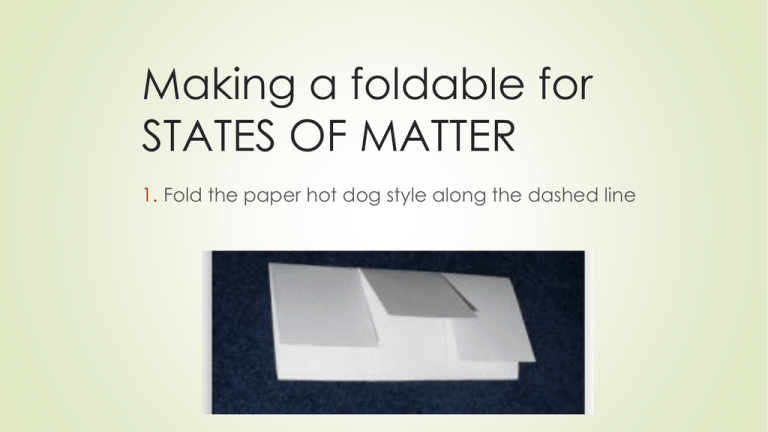
Making a foldable for STATES OF MATTER 1. Fold the paper hot dog style along the dashed line Flap 1- ? Solid These particles represent Atoms (if the solid is an element) or Molecules (if the solid is a compound) Flap #1- Speed of particles: solid How are the particles moving? Tightly packed particles DO NOT move past each other. They vibrate in place. © 2013 S. Coates The only time particles of matter do NOT move, is when the temperature is absolute zero (-459 F) Shape: solid Take the wooden block- place it in the beaker Now place it in the petri dish Can you fit it in the flask? (Don’t try, just think about it) What do you notice about the shape of a solid-does it change? Solids have a DEFINITE SHAPE Volume: solid Take the wooden block and place it in the beaker Now place it in the petri dish Could you get it into the flask? (Don’t try, just think about it) Does the block take up more or less space depending on its container? Has its volume changed? Solids have DEFINITE VOLUME Examples: Solids Wooden block Marble Add two more examples of solids on your own Flap 2- ? Liquid Flap #2- Speed of particles: liquid How are the particles moving in a liquid? Tightly packed particles SLIDE past each other. © 2013 S. Coates Shape: liquid Measure 20 mL of water into the graduated cylinder Pour it into the beaker. Use the funnel to pour it into the flask What do you notice about the shape of a liquid-does it change in each container? Liquids have NO DEFINITE SHAPE (they take the shape of the container) Volume: liquid Carefully pour the water from the flask back into the graduated cylinder How much water is in there? Have you added/subtracted any liquid to the water? Has its volume changed? Liquids have DEFINITE VOLUME Examples: liquids Water Juice Add two more examples of liquids on your own Flap 3 - ? Gas Flap #3- Speed of particles: gas How are the particles moving in a gas? Particles are not tightly packed together, and have so much energy they slip past each other quickly. © 2013 S. Coates Shape: Gas • Observe the following: Does the gas have the same shape in each container? • Gases have NO DEFINTE SHAPE (They take the shape of the container) Volume: Gas • Observe the following: Does the gas fill the container? • What if I change the size of the container? Does the gas take up a different amount of space? • Gases have NO DEFINTE VOLUME (They can expand or compress to fill the container) Think about spraying air freshener or body spray, this fragrant gas will expand to fill a room because gas has no definite volume! Examples: gas Air Smoke Add two more examples of gases on your own 4th Phase of Matter: Plasma Particles are moving so quickly it is hard to see what they are actually doing. © 2013 S. Coates Examples of Plasma on Earth © 2013 S. Coates Phases of Matter Let’s summarize: Phase Motion of Particles Speed of Particles Solid Particles vibrate in place Slow Liquid Particles are close, but can slide past one another Medium Gas Particles are constantly moving everywhere Fast Plasma Unknown Faster than we can see © 2013 S. Coates Physical Properties of Three States of Matter Phase Solid Liquid Gas Plasma © 2013 S. Coates Definite Shape? Definite Volume? YES YES NO YES NO NO Copper Phases - Solid Copper Phases - Liquid Copper Phases – Vapor (gas) What do we call it when matter changes from one state to the next? VAPORIZE MELT FREEZE CONDENSE SUBLIMATION 1. A Review of the States of Matter What phase of matter has the particles with the least amount of motion? Solid 2. A Review of the States of Matter What temperature do particles show NO motion? Absolute zero 3. A Review of the States of Matter What type of motion do particles have in a gas state? Very far apart and they move very quickly. 4. A Review of the States of Matter What properties do liquids and solids have in common? Definite volume 5. A Review of the States of Matter What properties do liquids and gases have in common? Both take on the shape of the container they are in. 6. A Review of the States of Matter Infer: A scientist places 25 mL of a yellow substance into a 50 mL container. The substance quickly fills the entire container. Is it a solid, liquid, or gas and why? Gas- takes the shape and volume of the container. 7. A Review of the States of Matter Why can I smell cookies baking in the kitchen when I am on the other side of the house? Gases have no definite volume and will fill the container they are in. 8. A Review of the States of Matter What is the fourth state of matter? Plasma 9. A Review of the States of Matter Which description best describes a solid? a. It has a definite shape and volume b. It has a definite shape but not definite volume c. It adjusts to the shape of its container d. It can flow A 10. A Review of the States of Matter In which state of matter do particles stay close together, yet are able to slip past one another? liquid So HOW does matter change its state? Phases of Matter • Energy is what changes a phase of matter. • Argon BOILS at -186°C, so when you hold it at room temperature you can see ALL 3 phases at the same time. © 2013 S. Coates What is energy? Energy is the ability to do work or cause change. Kinetic Energy Kinetic Energy is the energy of motion Particles with a lot of kinetic energy move fast and far apart Particles with little kinetic energy move slow & close together Particles with a lot of kinetic energy Particles with little kinetic energy Thermal Energy The total kinetic energy of all the particles in a sample of matter is called thermal energy. Temperature Temperature is the average kinetic energy of the individual particles in a substance So… if it is hot more kinetic energy, if cold less kinetic energy. Heat The movement of thermal energy from a substance at a higher temperature to one at a lower temperature is called heat. Changing states Matter can change from one state to another when thermal energy is released or absorbed. This is called a change of state. Melting The change from the solid state to the liquid state is melting. The temperature at which a substance changes from a solid to a liquid is called the melting point. Melting is when matter absorbs thermal energy, and its temperature rises. Freezing The change from the liquid state to the solid state is called freezing. The temperature at which a substance changes from the liquid state to the solid state is called the freezing point. Energy is released during freezing. After all of the liquid has become a solid, the temperature begins to decrease again. Vaporization The change from a liquid to a gas is known as vaporization. The temperature of the substance does not change during vaporization. However, the substance absorbs thermal energy. Vaporization Two forms of vaporization exist. Vaporization that takes place below the surface of a liquid is called boiling. The temperature at which a liquid boils is called the boiling point. Vaporization that takes place at the surface of a liquid is called evaporation. Evaporation • Evaporation, which occurs at temperatures below the boiling point, explains how puddles dry up. • It takes more than speed for water molecules to escape the liquid state. • During evaporation, these faster molecules also must be near the surface, heading in the right direction, and they must avoid hitting other water molecules as they leave. The opposite of vaporization is called condensation, which occurs when a gas loses enough thermal energy to become a liquid. The change of state from gas to liquid is called condensation. Condensation As a gas cools, its particles slow down. When particles move slowly enough for their attractions to bring them together, droplets of liquid form. This process, which is the opposite of vaporization, is called condensation. Sublimation • Some substances can change from the solid state to the gas state without ever becoming a liquid. During this process, known as sublimation, the surface particles of the solid absorbs enough energy to become a gas. Picture from http://www.ehow.com/how_2098268_fogsmoke-dryice-halloween.html Deposition Deposition moving directly from a gas to a solid state The opposite of sublimation State Change Pyramid Absorbing thermal energy Gas Releasing thermal energy Freezing Solid Melting Liquid Phases of Matter • ADDED Is ENERGY being ADDED or TAKEN AWAY in this phase change: The added energy has caused the chocolate particles to speed up. Before they were vibrating in place, now they are moving fast enough to slip past one another. Solid © 2013 S. Coates Liquid Phases of Matter • Is ENERGY being ADDED or TAKEN AWAY in this phase change: ADDED The added energy has caused the water particles to speed up. Before they were moving fast enough to slip past one another, now they have enough energy to break away from one another and expand. Liquid © 2013 S. Coates Gas Phases of Matter • Is ENERGY being ADDED or TAKEN AWAY in this phase change: Taken Away Taking away energy from a rain drop slows the water molecules down so that they no longer slide past one another. © 2013 S. Coates Liquid Solid http://www.youtube.com/watch?v=ghl33n 26d44 Iceland volcano clip