Cornell notes about heat and how heat transfers
advertisement

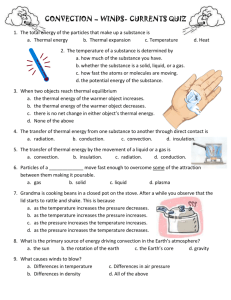
CORNELL NOTES ABOUT HEAT AND HOW HEAT TRANSFERS Essential Question #1: What is heat and how does it differ from temperature? Essential Questions #2: What are the methods of heat transfer and how does each one work? WHAT IS HEAT? • A form of energy that flows between two samples of matter because of the differences in temperature. Heat always flows from areas of HOT TO COLD. WHAT IS THE DIFFERENCE BETWEEN HEAT AND TEMPERATURE? • Heat is the total amount of energy transferred in systems due to a difference in temperature, while temperature is a measure of how fast molecules are moving in a substance (how much heat is present). Thermal energy is related to temperature. A substance can have thermal energy with no heat (because particles in atoms are always moving and this is thermal energy) 3 METHODS OF HEAT TRANSFER: • 1. Conduction- the transfer of heat from one molecule in a substance to another by means of DIRECT CONTACT of the molecules (occurs in solids). • 2. Convection- the transfer of heat in liquids or gases due to currents. These currents are caused by differences in density due to differences in temperature (creating up and down currents). • 3. Radiation- heat transfer that travels through space and can move THROUGH something. IMAGES OF EACH TYPE OF HEAT TRANSFER EXAMPLES OF EACH TYPE OF HEAT TRANSFER • CONDUCTION: -Touching a stove and being burned -Ice cooling down your hand -Boiling water by thrusting a red-hot piece of iron into it • -when computer on for long time, when your feet touch hot sand, when you touch a generator, when you touch a light bulb that gives off heat • Convection: when you turn on hot sink water boiling water hot spring water rising, warm air • RADIATION: -Heat from the sun warming your face -Heat from a lightbulb (not direct contact) -Heat from a fire -Heat from anything else which is warmer than its surroundings.