Connecting-the-unit-cell-structure-with-density
advertisement

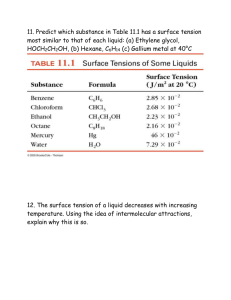
Connecting the unit cell structure with density Let's start by a) reviewing what density is – what units do we use? b) if we know what element we are using, what source can we use to find the mass? c) finding the connection between the unit cell, the atomic radius, and volume Simple cubic Face-Centered cubic Body-Centered cubic Sample problems 1. Iridium has a face-centered cubic unit cell with an edge length of 383.3 pm. a) Calculate the density of solid iridium. b) What is the atomic radius of iridium? c) What percentage of the unit cell is taken up by atoms? 2. Titanium metal has a body-centered cubic unit cell. The density of titanium is 4.50 g/mL. a) What is the edge length of this unit cell? b) What is the atomic radius of titanium? c) What percentage of the unit cell is taken up by atoms? 3. Polonium is one of the few metals that crystallizes in a simple cubic arrangement. It has an edge length of 334 pm. a) What is the density of solid polonium? c) What percentage of the unit cell is taken up by atoms? Practice problems: 1. The radius of a nickel atom is 124 pm. If it crystallizes with a face-centered cubic structure, what is the density of nickel? 2. The density of gold is 19.3 g/mL. Gold crystallizes in a face-centered cubic arrangement. What is the radius of a gold atom? 3. Potassium crystallizes in a body-centered cubic structure. What is the atomic radius of potassium if the unit cell edge length is 533.3 pm? 4. Barium has a body-centered cubic structure. If the atomic radius of barium is 222 pm, what is the density of solid barium?