Sulfur Cycle Britt and Olivia Oct 09
advertisement
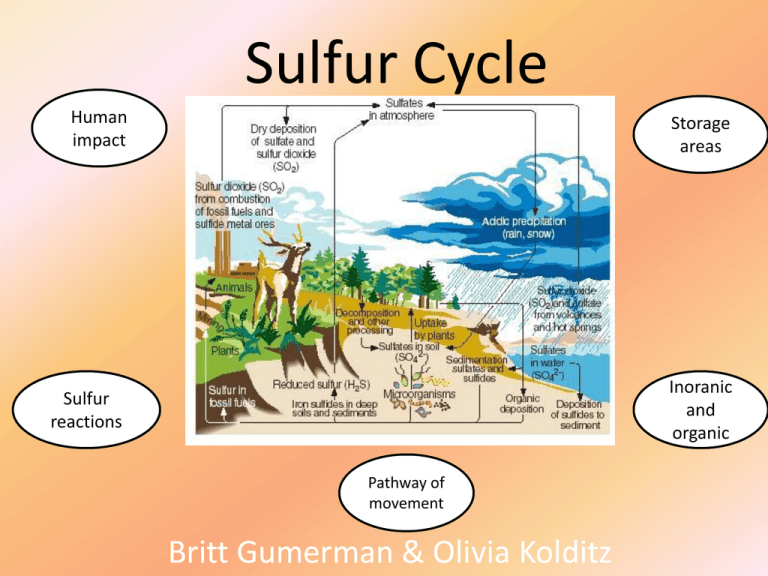
Sulfur Cycle Human impact Storage areas Sulfur reactions Inoranic and organic Pathway of movement Britt Gumerman & Olivia Kolditz Impact of Human Intervention Refining sulfur-containing petroleum to make gasoline, heating oil, and other useful products. Burning sulfurcontaining coal and oil to produce electric power. Since the Industrial Revolution, human activities have contributed to the amount of sulfur that enters the atmosphere. Using smelting to convert sulfur compounds of metallic minerals into free metals such as copper, lead, and zinc The sulfur released by humans such as sulfur dioxides and sulfur aerosols also results in acid rain Result of acid rain Reaction to sulfur with air Sulfur burns in air to form the gaseous dioxide sulfur(IV) oxide, SO2. S8(s) + 8O2(g) → 8SO2(g) Reaction of sulfur with water Sulfur does not react with water under normal conditions. Reaction of sulfur with the halogens Sulfur reacts with all the halogens upon heating. Sulfur reacts with fluorine, F2, and burns to form the hexafluoride sulfur(VI) fluoride. S8(s) + 24F2(g) → 8SF6(l) [orange] Reaction of sulfur with acids Sulfur does not react with dilute non-oxidizing acids. Reaction of sulfur with bases Sulfur reacts with hot aqueous potassium hydroxide, KOH, to form sulfide and thiosulphate species. S8(s) + 6KOH(as) → 2K2S3 + K2S2O3 + 3H2O(l) 1)Terrestrial rocks 2) Ocean sediments Terrestrial rock Ocean sediments •Sodium dithionite, Na2S2O4 •Thioethers •Thiosulfates (S2O32−) •Sulfonium •Thiols •Sulfides (S2−) •Sulfites (SO32−) •Thiolates •Sulfoxides •Sulfones •Sulfates (SO42−) Located in acid deposits Located in Earth’s crust