The Sulfur Cycle
advertisement

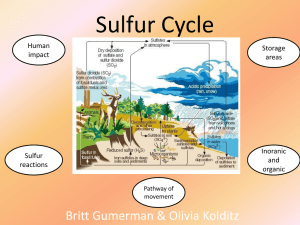
The Sulfur Cycle Aslan Smith James Littrell Olivia Stephens Kristina Pesce WHAT is sulfur? Atomic number: 16. Symbol: S Native form: is a yellow crystalline (crystal like) solid. In nature: it can be found as the pure element, and as sulfide and sulfate minerals. commercial uses: fertilizers, gunpowder, matches, insecticides, fungicides, vitamins, proteins and hormones. It is critical in the environment, climate and the health of ecosystems. Random facts: it can also be referred to as brimstone. it’s the tenth most abundant element in the universe WHERE is sulfur found? The majority of Earth's sulfur is stored: In rocks underground! In sulfur salts at the bottom of the ocean! The Cycles: Erosion, weathering, deposition Predominately atmospheric cycle Marine cycle Soil-plant cycle The Cycles: part two!! Mineralization of organic sulfur to the inorganic form hydrogen sulfide (H2S). Oxidation of sulfide and elemental sulfur (S) and related compounds to sulfate (SO4). Reduction of sulfate to sulfide Microbial immobilization of the sulfur compounds and subsequent incorporation into the organic form of sulfur! The Terrestrial Portion Weathering of rocks release stored sulfur Sulfur comes into contact with air and is converted into sulfate (SO4) ions The sulfate is taken up by plants and microorganisms, converted into organic forms Animals consume organic molecules containing sulfur, sulfur moves through the food chain The death and decomposition of organisms release sulfur once again in sulfate form and some of it becomes part of the biomass of microorganisms. The Atmospheric Portion Volcanic eruptions, breakdown of organic matter in swamps and tidal flats, and the evaporation of water, especially seawater, release sulfur directly into the atmosphere Sulfur eventually settles to earth or comes down with rainfall In the Oceans In oceans, sulfur moves through the various marine food webs Some sulfur is lost in the ocean by being incorporated into ferrous sulfide and settling to the seafloor Human Activities The burning of fossil fuels and processing of metals releases huge quantities of sulfur into the atmosphere Human activities are responsible for one-third of all sulfur emissions and 90% of all sulfur dioxide emissions Sulfur dioxide emissions lead to acid rain as sulfur dioxide reacts with water to form H2SO4 and sulfur trioxide reacts with water to form H2SO4 READY FOR A POP QUIZ? Flip to page three on your hand out. You will be given one minute to complete the following questions! Good luck!! Answers! Vitamins, proteins, fertilizers (others?) Volcanoes! (others?) The weathering of rocks! (deposition) Terrestrial/Atmospheric (Marine is also acceptable) Humans! 1/3rd in fact! Sources: http://www.enviroliteracy.org/article.php/1348.html http://www.atmosphere.mpg.de/enid/Nr_6_Feb__2__ 6_acid_rain/C__The_sulphur_cycle_5i9.html http://www.cliffsnotes.com/WileyCDA/CliffsReviewTo pic/The-Sulfur-Cycle.topicArticleId-23791,articleId23787.html