13. Mass spectrometry in organic chemistry AND 14. fragmentation
advertisement

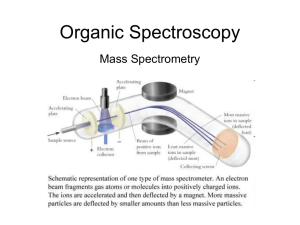
Mass spectrometry in organic chemistry Starter: Use mini wipe boards to brainstorm what you know about mass spectrometry Keywords: Molecular ion (M+) Fragmentation Learning Objectives A: Use the molecular ion peak in an organic molecule’s mass spectrum to determine its molecular mass. B: Explain that a mass spectrum is essentially a molecule’s fingerprint that can be identified using a spectral database. Molecular ion (M+) – the positive ion formed when a molecule loses an electron Fragmentation – the process that causes a positive ion to split into pieces, one of which is a positive fragment ion Mass Spectrometry When an organic compound is placed in a mass spectrometer, some molecules lose an electron and are ionised. The resulting positive ion is called the molecular ion and is given the symbol M+ Electron impact is the oldest and best established method of ionisation. The molecules being tested are bombarded with electrons. If an electron is dislodged from a molecule, a positive ion is formed. With ethanol, a C2H5OH molecule is ionised to form the molecular ion, C2H5OH+ C2H5OH + e- C2H5OH (molecular ion) + 2e- Mass Spectrometry • The mass of the electron is negligible • The molecular ion has a molecular mass equal to the molecular mass of the compound • This molecular ion can be detected and analysed Excess energy form the ionisation process can be transferred to the molecular ion, making it vibrate. This causes bonds to weaken and the molecular ion can split into pieces by fragmentation. Fragmentation results in a positive fragment ion and a neutral species. Mass Spectrometry A possible fragmentation of an ethanol molecular ion; C2H5OH+ CH3 + CH2OH+ (fragment ion) Fragment ions are often broken up further into smaller fragments. The molecular ion and fragment ions are detected in a mass spectrometer. The molecular ion (M+) produces the peak with the highest m/z value in the mass spectrum Mass Spectrometry The molecular mass of a molecule can be determined using mass spectrometry by locating the M+ peak For ethanol, the molecular ion peak is located at a m/z ratio of 46. this indicates that the molecular mass of the molecule is 46. the other peaks in the spectrum are a result of fragmentation Mass Spectrometry Mass spectrometry can be used to determine the structure of an unknown compound. • Although the molecular ion peak of 2 isomers will have the same m/z value, the fragmentation patterns will be different • Each organic compound produces a unique mass spectrum, which can be used as a fingerprint for identification Look at the mass spectra for pentane and 2methylbutane. Identify the M+ ion peak and fragmentation patterns Mass Spectrometry Mass Spectrometry Although mass spectra can be analysed by viewing the spectra, modern mass spectrometers are often linked to a special database. When a mass spectrum for an unknown sample is produced, the spectral database is scanned automatically until a perfect match is found Plenary 1. Write a balanced equation showing the ionisation for octane. State the likely m/z value for the molecular ion peak 2. In the spectra for compounds A and B, identify the m/z value for each molecular ion peak Mass spectrometry: fragmentation patterns Starter: ???? Keywords: Fragment ion Learning Objectives A: Suggest the identity of the major fragment ions in a given mass spectrum. B: Use molecular ion peaks and fragmentation peaks to identify structures. Fragment ion – a positive ion produced by the splitting of a molecular ion Mass Spectrometry When looking at a mass spectrum, fragment peaks appear alongside the more important molecular ion peak. These fragment peaks give information about the structure of the compound. Some common peaks to look out for: m/z value 15 17 29 43 57 Possible identity of fragment ion CH3+ OH+ C2H5+ C3H7+ C4H9+ Mass Spectrometry As well as being used to identify the molecular mass of a molecule, a mass spectrum can also be used to work out some of the molecule’s structural detail. Use the mass spectrum to identify the alkane Mass Spectrometry The equations below show how the molecular ion could be fragmented to form fragment ions with m/z values of 57 and 43 Plenary 1. The mass spectrum is for pent-1-ene. Suggest the ions responsible for the peaks labelled A, B and C. 2. Draw structures for the fragment ions represented by peaks with m/z values of 43, 57 and 71