Chapter 19 PPT - Saluda County School District 1
advertisement

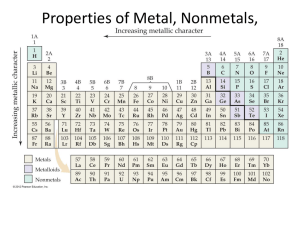
Chapter 19 Elements and Their Properties 19.1 Metals Properties of Metals • Metals have common properties • • • • • Good conductors of heat and electricity All but Mercury are solid at room temperature Reflect light – Luster Easily hammered/rolled into sheets – Malleable Easily drawn into wires - Ductile Ionic Bonding • Metal atoms have usually 1-3 valence electrons • Tend to give their electrons up easily • When they combine with nonmetals, they lose electrons to the nonmetals, forming ionic bonds • This makes them more chemically stable Metallic Bonding • Occurs among metal atoms • Positive charged metallic ions are surrounded by a cloud of electrons • The electrons move freely among many different positively charged metal ions • Explains many properties of metals • Hammered • Good conductors Alkali Metals • Group one • Properties • • • • • Shiny Malleable Ductile Softer Most reactive of all the metals – react rapidly/violently w/ oxygen and water • Don’t occur in elemental form in nature – stored in oil Alkali Metals • One electron in its outer energy level • The atom gives up this electron with it combines with another atom ex. NaCl • Living things need alkali metals/their compounds • K, Na, Li Radioactive Element – one in which the nucleus breaks down and gives off particles and energy ex. Francium – very rare & radioactive Alkaline Earth Metals • Group 2 • Also not found as free elements in nature – combine so readily with other elements • 2 electrons in outer energy level • These are given up with they combine with nonmetals and becomes a positively charged ion in a compound such as CaF2 Alkaline Earth Metals • Magnesium & Strontium – used in fireworks • Chlorophyll – Magnesium compound – enables plants to make food • Magnesium’s lightness & strength • Cars, planes, spacecraft • Household ladders, baseball/softball bats Alkaline Earth Metals • Calcium compounds needed for life • Calcium phosphate – makes bones strong • Barium compound – swallowed to take x-rays to diagnose internal abnormalities • Radium once used to treat cancer • They now use other readily available radioactive elements Transition Elements • Elements in Groups 3-12 • Often occur in nature as uncombined elements b/c they are more stable • Often form colored compounds Iron, Cobalt, Nickel • Called the Iron Triad • Used to create steel/other metal mixtures • Nickel – added to some metals to make them stronger or give them a shiny protective coating • Iron – main component of Steel – most widely used of all metals • Second most abundant metallic element in Earth’s crust (Al is 1st) Copper, Silver, Gold • Very stable and malleable • Found as free elements in nature • Copper often used in electrical wiring • Silver compounds – photographs, film • Silver & Gold – used in jewelry • Once used to make coins – termed the coinage metals • Not anymore b/c $$$, most coins now are Ni and Cu Zinc, Cadmium, Mercury • Zn, Cd often used to coat other metals • Cd used in rechargeable batteries • Hg – silvery, liquid metal • Used in thermometers, thermostats, switches, batteries • Poisonous – can accumulate in body Inner Transition Metals • Lanthanides – follow the element lanthanum • Used with carbon to make compound used to make movies • Used to produce color in TV screens Inner Transition Metals • Actinides – second row • Follow the element actinium • All radioactive and unstable • Used to make high quality camera lenses, nuclear reactors, weapons Questions 1. How is metallic bonding different from covalent bonding (when two elements share electrons) 2. How would you test something to see if it is a metal? 3. Why is mercury rarely used in thermometers that take body temperature? 4. Explain why copper is a good choice for use in electrical wiring. What type of elements would not work well for this purpose? 19.2 Nonmetals Properties of Nonmetals • Nonmetals – elements that are usually gasses or brittle solids at room temperature • Not malleable or ductile • Most don’t conduct well • Generally not shiny • All nonmetals except hydrogen are found to the right of the stair step line Bonding in Nonmetals • Electrons in most nonmetals are attracted to the nucleus of the atom, so they are poor conductors • Can form ionic or covalent bonds • When nonmetals get electrons from metals, they become the NEGATIVE ions in ionic compounds • When bonding w/ other nonmetals, usually share electrons to form covalent compounds Hydrogen • 90% of atoms in the universe are hydrogen • Most on earth found in water When water is broken down, H becomes a gas made up of diatomic molecules Diatomic molecule – 2 atoms of the same element in a covalent bond Hydrogen • Highly reactive • Single electron – shared when combined with other nonmetals • Hydrogen can gain an electron when combining with alkali and alkaline earth metals to form a hydride ex. NaH (sodium hydride) Halogens • Group 17 • Very reactive in elemental form • Compounds have many uses • Fluorides – toothpaste, city water, water to disinfect • 7 electrons in outer energy level, so only need 1 to be HAPPY ☺ Halogens • If a halogen gains an electron from a metal, forms an ionic compound called a Salt ex. NaCl • In gas state, halogens form reactive diatomic covalent molecules – can be identified by colors • Chlorine – greenish yellow • Bromine – reddish orange • Iodine - violet Halogens • Fluorine – most chemically active of all elements • Uses of Halogens • Chlorine compounds – distinct small, most abundant halogen, used in bleaches to whiten things • Bromine – only liquid nonmetal at room temp., used in dyes in cosmetics • Iodine – shiny solid at room temp, when heated changes directly to purple vapor – sublimination • Essential to diet to produce certain hormones Noble Gases • Stable • Outermost energy levels full Neon and Argon - neon lights Lightweight helium used in balloons Argon and Krypton used in electric light bulbs to produce light in lasers