Transition Metals & Coordination Compounds
advertisement

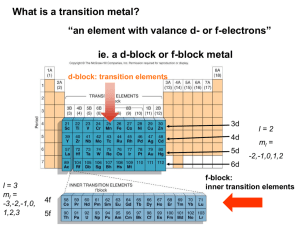
Chapter 24 Transition Metals & Coordination Compounds • 24.2 Properties of Transition Metals o Review Electron Configuration o Trends in the Periodic Table • 24.3 Coordination Compounds o The Basics o Example of Naming • 24.4 Structure and Isomerization Transition Metals contain e- in d Orbitals http://www.can-do.com/uci/lessons98/Periodic.html http://www.can-do.com/uci/lessons98/Periodic.html Why Are Transition Metals & Coordination Compounds Important? • Therapeutic drugs • Chemical Sensors • Coloring agents o Paints o Cosmetics • Biological Molecules o Hemeglobin o Chlorophyll • Gems (Jewelry & Technological Applications) o Rubies, Emeralds, Garnets, etc. o Lasers 24.2 Properties of Transition Metals • Moderate to High Densities • Good Electrical Conductivity • High Melting Points • Moderate to Extreme Hardness Due to the delocalization of d electrons in metallic bonding Exceptions: Elements with filled d orbitals, which prevents d-d bonding. Hg has a low melting point and is liquid at room temperature. http://www.tutorvista.com/chemistry/shapes-of-d-orbitals Electron Configuration I n c r e a s i n g E n e r g y (n-1)d (n-2)f Electron Configuration [noble gas] ns2 (n-1)dx [noble gas] ns2 (n-2)f14 (n-1)dx Electron Configuration [Kr] 5s2 4d2 http://malaxoschemistry.wikispaces.com/Periodic+Table Atomic Size I n c r e a s I n g Decreasing Size S i z e http://malaxoschemistry.wikispaces.com/Periodic+Table Atomic Size Exception to the trend: Electrons in the f-orbitals are not effective at shielding outer shell electrons from nuclear charge. So, the outer electrons are held in close – this is known as lanthanide contraction. Ionization Energy Increases D e c r e a s e s http://malaxoschemistry.wikispaces.com/Periodic+Table Ionization Energy Exception to the trend: Note that 5d elements have a greater ionization energy. This is again due to outer shell electron being held closer to the nucleus, so it take more energy to pull them away. Electronegativity Increases D e c r e a s e s http://malaxoschemistry.wikispaces.com/Periodic+Table Electronegativity Au: EN = 2.4 Compared to P: EN = 2.1 !! Exception to the trend: There is an increase in electronegativity from the 3d (1st row transition metals) to the 4d (2nd row transition metals). Oxidation States In general, stability is found in full or halffull shells, and in a configuration that looks like a noble gas. 24.3 Coordination Compounds • Complex Ion - Central Metal bound to one or more ligands • Ligands are Lewis Base* (electron donors) and can be either neutral or negatively charged • The charge on the complex ion is balance by counter ions of opposite charge The combination of a complex ion and counter ions results in a coordination compound *Corrected 4/15/11 @ 2:30 pm) David N. Blauch - http://www.chm.davidson.edu/vce/index.htm A Little Background • In 1893, Swiss chemist Alfred Werner came up with the idea that a central metal could have 2 types of interactions o Primary Valence – Oxidation State of the central metal o Secondary Valence – Number of molecules or ions directly attached to the central metal or Coordination Number • Example: [Co(NH3)6]Cl3 o The Primary Valence or Oxidation State of Co is +3 o The Secondary Valence or Coordination Number is 6 (6 ammonia ligands are directly attached to Co • Other cobalt(III) coordination compounds • [Co(NH3)6]Cl3 • [Co(NH3)5Cl]Cl2 • [Co(NH3)4Cl2]Cl Coordinate Covalent Bonds • Lewis Acid-Base Adduct – the ligand donates it’s electrons to the empty metal orbitals to form a coordinate covalent bond M : L Lewis Base Lewis Acid Adduct Some Common Ligands Chelating Agents • Ligands can have one or more bonding pairs of electrons o Monodentate o Bidentate or Polydentate • Complex ions with bidentate or polydentate ligands are chelates, and the coordinating ligands are chelating agents Co EDTA is hexadentate http://library.kiwix.org:4201/A/Inorganic_chemistry.htm Geometries Anne Marie Helmenstine, Ph.D., About.com Guide Naming Coordination Compounds • [Mn(CO)(NH3)5]SO4 (neutral ligands are written before charged ligands in the formula) • Cation 1st o Name the ligands in alphabetical order • ammine • carbonyl o Add a prefix to indicate the number of ligands • pentaammine o Name the metal ion • Manganese(II) • Anion 2nd o Sulfate • Pentaamminecarbonylmanganese(II) sulfate 24.4 Structure & Isomerism Isomers Same formula – different structures Structural Isomers Stereoisomers Different connectivities Same connectivities –different spacial arrangements Coordination Isomers Linkage Isomers Ligands & counter ions trade places Ligands coordinate in different ways Geometric Isomers Optical Isomers Different spacial arrangements Mirror images cis-trans fac-mer Structural Isomers Coordination Isomers pentaamminesulfatochromium(III) bromide pentaamminebromochromium(III) sulfate David N. Blauch - http://www.chm.davidson.edu/vce/index.htm Structural Isomers Linkage Isomers pentaamminenitrocobalt(III) ion David N. Blauch - http://www.chm.davidson.edu/vce/index.htm pentaamminenitritocobalt(III) ion Stereoisomers Geometric Isomers: cis-trans cis trans cis-diamminedichloroplatinum(II) trans-diamminedichloroplatinum(II) David N. Blauch - http://www.chm.davidson.edu/vce/index.htm Stereoisomers Geometric Isomers: fac-mer fac fac-triamminetrichlorocobalt(III) David N. Blauch - http://www.chm.davidson.edu/vce/index.htm mer mer-triamminetrichlorocobalt(III) Stereoisomers Optical Isomers • • • • Mirror Images Non-superimposable Enantimomers Chiral: optically active (rotates polarized light) http://en.wikipedia.org/wiki/Chirality_%28 electromagnetism%29 http://www.wikidoc.org/inde x.php/Chirality_%28chemist ry%29 Chirality Determining Optical Activity fac mer David N. Blauch - http://www.chm.davidson.edu/vce/index.htm Chirality Determining Optical Activity Superimposable No optical activity