Optical Isomers
advertisement

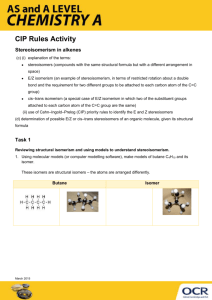
Do Now Take out your worksheet (work in group first ) Objective Doing the problems from the worksheet (for going over the naming method) Saturated compounds /Unsaturated compounds Comparison between Alkane ;Alkene ;Alkyne Isomers Homework P 719/43,44,45,48 Answer for worksheet 1. 2. 3. 4. 5. 6. 7. 2-methyl propane 2-ethyl propane 2-ethyl heptane 3-ethyl hexane 2,2,4-trimethyl Pentane 3-methyl-3-Hexyl Heptane 4-ethyl-6-methyl nonane • Saturated compounds • Concept : Organic compounds that contain the maximum number of hydrogen atoms per carbon atom • Example : Alkanes • Unsaturated compounds • Concept : Compounds that contain double or triple carbon-carbon bonds • Example : Alkenes ;Alkynes Guessing about bonds Every bond means two atoms sharing two electrons together • Alkanes • The only bonds in alkanes are single covalent bonds • CnH2n+2 Ethane Ethene • Alkynes • Hydrocarbons that contain one or more carboncarbon triple covalent bonds. • CnH2n-2 • Alkenes • Hydrocarbons that contain one or more carboncarbon double covalent bonds • CnH2n Ethyne Name Molecular structure Boiling point (Casers) Ethane CH3--CH3 -88.5 Ethene CH2== CH2 -103.9 C2 Ethyne -81.8 C3 Propane CH3CH2CH3 -42.0 Propene CH3CH==CH2 -47.0 Propyne -23.3 The introduction of a double or triple bond into a hydrocarbon does not have a dramatic effect on physical properties such as boiling point. But the double bonds is more stable ,so it needs more energy to break it apart. Naming Alkene To name an alkene by the IUUPAC system , find the longest chain in the molecule that contains the double bond. This chain is the parent alkene It uses suffix –ene . The chain is numbered so that the carbon atoms of the double bond have the lowest possible number Substituents on the chain are named and numbered in the same way they are for the alkanes. Draw ,name all the alkenes with the molecular formula C4H8 Identify and name the alkanes ; alkenes ;alkynes C 4 H8 Alkene Butene C 5 H8 Alkyne Pentyne C 3 H8 Alkane Propane C 3 H6 Alkene Propene C6H10 Alkyne Hexyne C4H10 Alkane Butane Tell which is saturated and unsaturated C 4 H8 C 3 H8 C6H10 C 5 H8 C 3 H6 C4H10 Alkanes are saturated compounds . Because the only bonds in it are single covalent bonds. • Isomers-- Compounds that have the same molecular formula but different molecular structures • Structural isomers • Stereoisomers • Geometric Isomers • Optical Isomers Isomers Structural Isomers Stereoisomers Geometric Isomers Optical Isomers Structural Isomers: • same molecular formula , different order. • differ in physical properties. different chemical relativities. Stereoisomers • Molecules in which the atoms are joined in the same order, but the positions of the atoms in space are different. • Two types : Geometric Isomers and optical Isomers Geometric Isomers have atoms joined in the same order , but differ in the orientation of groups around a double bond. Optical Isomers are that pairs of molecules that differ only in the way that four different groups are arranged around a central carbon atom . Geometric Isomers same order , differ in the orientation of groups around a double bond. Trans Configuration The methyl groups are on opposite sides of the double bond Cis Configuration The methyl groups are on the same side of the double bond Optical Isomers are that pairs of molecules that differ only in the way that four different groups are arranged around a central carbon atom . Asymmetric carbon is a carbon with four different atoms or groups attached Draw a structure formula or carbon skeleton for each of the following alkenes .If cis and trans forms are present ,include both forms. a.2-pentene b.2-methy-2-pentene c.3-ethyl-2-pentene Draw one structural isomer of each compound P 719/43,44,45,48