*Forensic hydrology*:
advertisement
BEE 3710
Spring 2011
using tracers to investigate
hydrology and biogeochemistry
Dissolved
constituents, isotopes,
particles or physical properties of
water that are used to track the
movement of water through
watersheds
Source: USGS circular 1139
http://pubs.usgs.gov/circ/circ1139/
naturally
occurring (e.g. chloride, silica, stable
isotopes, organic compounds)
artificial or researcher introduced (e.g. various
dyes, plastic microspheres)
sometimes unintentionally introduced! (e.g.
tritium, chlorofluorocarbons, certain radioactive
isotopes)
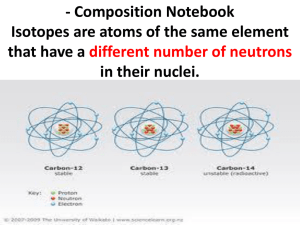
a less common isotopic form of an element
physical property of water (e.g. temperature)
General: Used to identify flow paths, travel times,
etc.
Specific uses:
Subsurface processes
e.g. preferential flow, groundwater movement
Surface
processes
Biogeochemical interactions
e.g. biological nitrogen uptake
pollutants
sediment
nutrients
Image source: http://www.twp.west-bloomfield.mi.us
Same
# of protons and electrons; different #
neutrons, so different masses!
Some isotopes not very dominant
Represented
as ‘delta’ or ‘per mil’
δ(in ‰) = (Rsample/Rstandard - 1)1000
A
where "R" is the ratio of the heavy to light
isotope in the sample or standard
positive δ value means that the sample
contains more of the heavy isotope than the
standard; a negative δ value means that the
sample contains less of the heavy isotope
than the standard
Fractionation:
when the relative amounts of
a particular isotope change due to the mass
differences
ie: lighter H & O isotopes are preferentially
evaporated
Equilibrium
vs Kinetic fractionation
Equilibrium: redistribution occurs, but reaction
rates same for forward/backward direction
Kinetic: reaction rates not same if products
become isolated from reactants
(SAHRA)
(SAHRA)
(Bowen et al 2006)
Useful
in surface and groundwater studies
In subsurface, useful for investigating
infiltration patterns, flow patterns for
contaminants
In streams, useful for quickly evaluating
travel time & mixing
Low
toxicity
High visibility
Consistent absorbance spectrum
(Flury & Wai 2003)
(Flury & Wai 2003)
Types:
Conservative
Don’t react biologically or strongly sorb to sediment
ie: bromide, chloride
Reactive
Compounds affected by biological and physical reactions
ie: NO3-
Studying
flood effects on stream interactions
Hyporheic
flow from woody debris
stream
FLOW
sediment
SUBSURFACE FLOW
(Bohlke et al 2004)
Fit
model to N2/N2O data
(Ritchie & McHenry 1990)
(Ritchie & McHenry 1990)
(Zhang & Walling 2005)
(Walling 2006)
DNA for identification
(and ability to have
multiple “tags”)
Magnetic iron oxide
nanoparticles to enable
capture
Polylactic acid forms
the framework
DNA: a polymer of four types of monomers (A, T, C, G)
Tracer of length m:
X1 X2 X3X4 …Xm
Xi = {A, T, C, G}
Number of potential tracers = 4m
1
4
Collection
point
1.05 m
2.20 m
2.85 m
Inlet
Tracer 2
Tracer 1
Outlet
Simple
Tracer 1
Tracer 2
modified
one dimensional
advection
dispersion model
with a dispersion
coefficient of
0.005 m2/s and a
loss factor of 6.6:
http://www.csrees.usda.gov/newsroom/partners/21/flow.html
Excess
phosphorus applied as fertilizer can
end up in streams and lakes in the watershed
Phosphorus can be sorbed on sediment on on
colloidal (<0.45 um ) particles/ dissolved in
water
If we want to know where the P is coming
from….sediment tracing works for P sorbed
on sediment, but what about dissolved P?
P + biomarker
B
P + biomarker A
+ biomarker B
P + biomarker A
(Fanelli& Lautz 2008)
18O
/2H
dye
Cs137
biomarkers
CFCs/
3H
bromide
N15
Tracers help figure out what’s going on in a complicated world!