CH17
advertisement

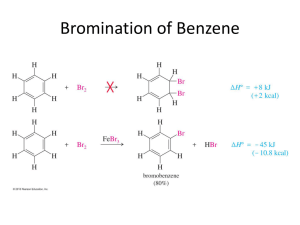
Organic Chemistry, 6th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17 2 Mechanism Step 1: Attack on the electrophile forms the sigma complex. Step 2: Loss of a proton gives the substitution product. => Chapter 17 3 Bromination of Benzene • Requires a stronger electrophile than Br2. • Use a strong Lewis acid catalyst, FeBr3. Br Br FeBr3 Br H + Br H Br FeBr3 H H H + H Br - H H Br FeBr3 + H _ + FeBr4 H H H Br + Chapter 17 HBr 4 Chlorination and Iodination • Chlorination is similar to bromination. Use AlCl3 as the Lewis acid catalyst. • Iodination requires an acidic oxidizing agent, like nitric acid, which oxidizes the iodine to an iodonium ion. Chapter 17 5 Nitration of Benzene Use sulfuric acid with nitric acid to form the nitronium ion electrophile. NO2+ then forms a sigma complex with benzene, loses H+ to form nitrobenzene. Chapter 17 6 Sulfonation Sulfur trioxide, SO3, in fuming sulfuric acid is the electrophile. Chapter 17 7 Nitration of Toluene • Toluene reacts 25 times faster than benzene. The methyl group is an activating group. • The product mix contains mostly ortho and para substituted molecules. => Chapter 17 8 Sigma Complex Intermediate is more stable if nitration occurs at the ortho or para position. => Chapter 17 9 Activating, O-, PDirecting Substituents • Alkyl groups stabilize the sigma complex by induction, donating electron density through the sigma bond. • Substituents with a lone pair of electrons stabilize the sigma complex by resonance. Chapter 17 10 Substitution on Anisole Chapter 17 11 The Amino Group Aniline, like anisole, reacts with bromine water (without a catalyst) to yield the tribromide. Sodium bicarbonate is added to neutralize the HBr that’s also formed. => Chapter 17 12 Summary of Activators => Chapter 17 13 Deactivating MetaDirecting Substituents • Electrophilic substitution reactions for nitrobenzene are 100,000 times slower than for benzene. • The product mix contains mostly the meta isomer, only small amounts of the ortho and para isomers. • Meta-directors deactivate all positions on the ring, but the meta position is less deactivated. => Chapter 17 14 Ortho Substitution on Nitrobenzene => Chapter 17 15 Para Substitution on Nitrobenzene => Chapter 17 16 Meta Substitution on Nitrobenzene => Chapter 17 17 Structure of MetaDirecting Deactivators • The atom attached to the aromatic ring will have a partial positive charge. • Electron density is withdrawn inductively along the sigma bond, so the ring is less electron-rich than benzene. => Chapter 17 18 Summary of Deactivators => Chapter 17 19 More Deactivators => Chapter 17 20 Halobenzenes • Halogens are deactivating toward electrophilic substitution, but are ortho, para-directing! • Since halogens are very electronegative, they withdraw electron density from the ring inductively along the sigma bond. • But halogens have lone pairs of electrons that can stabilize the sigma complex by resonance. => Chapter 17 21 Sigma Complex for Bromobenzene Ortho and para attacks produce a bromonium ion and other resonance structures. No bromonium ion possible with meta attack. => Chapter 17 22 Summary of Directing Effects Chapter 17 23 => Multiple Substituents The most strongly activating substituent will determine the position of the next substitution. May have mixtures. Chapter 17 24 Friedel-Crafts Alkylation • Synthesis of alkyl benzenes from alkyl halides and a Lewis acid, usually AlCl3. • Reactions of alkyl halide with Lewis acid produces a carbocation which is the electrophile. • Other sources of carbocations: alkenes + HF, or alcohols + BF3. Chapter 17 25 Limitations of Friedel-Crafts • Reaction fails if benzene has a substituent that is more deactivating than halogen. • Carbocations rearrange. Reaction of benzene with n-propyl chloride and AlCl3 produces isopropylbenzene. • The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. => Chapter 17 26 Friedel-Crafts Acylation • Acyl chloride is used in place of alkyl chloride. • The acylium ion intermediate is resonance stabilized and does not rearrange like a carbocation. • The product is a phenyl ketone that is less reactive than benzene. => Chapter 17 27 Mechanism of Acylation Chapter 17 28 End of Chapter 17 Chapter 17 29