B.5 * B.6 Notes
advertisement

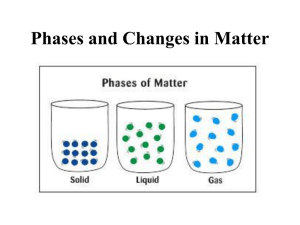
In which you will learn about: •Modeling atoms & molecules •Chemical symbols & formulas We live in a macroscopic world • Large-scale, readily observed things Chemistry explains what we observe in the macroscopic world by describing the interactions in the microscopic (particle) world Scientists use models to show objects that are either really big or really small • Models can also be used as simplifications of complicated ideas It is useful to use models of atoms & molecules to “see” chemistry happening Draw a model of two gaseous compounds in a homogeneous mixture. • A homogeneous mixture is uniform throughout, so the two compounds should be intermingled and evenly distributed. • Compounds are composed of atoms of two or more different elements linked together by chemical bonds. An international “chemical language” for use in oral and written communication was developed to represent atoms, elements, and compounds • The letters in this language’s alphabet are chemical symbols which are understood by scientists throughout the world Each element is assigned a chemical symbol • Only the first letter of the symbol is capitalized • All other letters are lowercase All known elements are organized in the periodic table of elements You are responsible for memorizing all of the elements and symbols on the Elements & Symbols sheet found in the Reference Sheet section of the Honors Page • Each set will be tested through quizzes Words in the language of chemistry are composed of letters (which represent elements) from the periodic table • Each word is a chemical formula, which represents a different chemical substance • A subscript (a number written below the normal line of letters) indicates how many atoms of the element just to the left of the subscript are in one unit of the substance H2O 2 atoms of hydrogen 1 atom of oxygen The chemical formula for propane, a compound commonly used as a fuel, is C3H8. What elements are present in a molecule of propane, and how many atoms of each element are there? Answer: 3 hydrogen atoms of carbon, 8 atoms of If formulas are words in the language in chemistry, then chemical equations can be regarded as chemical sentences. • Each chemical equation summarizes the details of a particular chemical reaction Chemical reactions entail the breaking and forming of chemical bonds, causing atoms to become rearranged into new substances. The new susbtances formed from a chemical reaction have different properties than those of the original materials • Starting material = reactant • New substances formed = products An arrow () represents the word “yield” • Reactants are on the left of the arrow • Products are on the right of the arrow Two hydrogen molecules react with one oxygen molecule to yield two molecules of water 2 H2 + O2 2 H2O The large number out front of a formula is called a coefficient • Coefficients represent the number of molecules of that type Perhaps you noticed that in the chemical equation on the previous slide, hydrogen and oxygen had two atoms in their molecule • These are examples of diatomic molecules There are 7 diatomic elements that must be memorized: hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, iodine “GEN – U –INE DIATOMICS” serves as a useful memory device for all common diatomic elements. • The names of all diatomic elements end in GEN or INE and U should remember them! 1) Sketch a visual model on the molecular level that represents each of the following types of mixtures. Label and explain the features of each sketch. • A. a solution B. a suspension 2) What two pieces of information does a chemical formula provide? 3) Name the elements and list the number of each atom indicated in the following substances: • A. phosphoric acid, H3PO4 (used in soft drinks) • B. Sodium hydroxide, NaOH (found in some drain cleaners) • C. sulfur dioxide, SO2 (an air pollutant) 4) Represent each chemical equation by drawing the molecules and their component atoms. Use circles of different sizes, colors, or shading for atoms of each element. In H2O2, the oxygens are bonded in the middle with hydrogens on the outside. • A. H2 (g) + Cl2 (g) 2 HCl (g) • B. 2 H2O2 (aq) 2 H2O (l) + O2 (g) • C. Using complete sentences, write word equations for the chemical equations given in A and B. Include the numbers of molecules involved.