TEST
advertisement

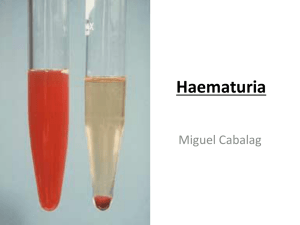
Introduction the routine urinalysis includes chemical testing for: pH ,protein, glucose, ketones, occult blood, bilirubin, urobilinogen, nitrite, leukocyte esterase, and strip test method for specific gravity Compound of urine :nitrogen compound of urine :1-urea 2- uric acid 3- creatinine . Non nitrogen compound of urine :1-vitamins 5-phosphate 2-amino acids 6-sulfate 3-chloride 7- calcium 4-potassium 8-magnesium Reagent strip consist of chemical-impregnated absorbent pads attached to the plastic strip. A colorproducing chemical reaction takes place when the absorbent pad comes in contact with urine. Reagent strip is the most common in chemical examination of urine………why??????? simple, rapid means for performing 10 medically significant chemical analyses, including pH, protein, glucose, ketones, blood, bilirubin, urobilinogen, nitrite, specific gravity video for reagent strips YnJGo9http://youtu.be/CPu-N :…Reagent Strips Procedu10 -neercShtlaeH ™Urin proceduer Sources of Error for reagent srtip: 1) Testing cold specimens - would result in a slowing down of reactions; test specimens when fresh or bring them to RT before testing 2) Inadequate mixing of specimen - could result in false reduced or negative reactions to blood and leukocyte tests; mix specimens well before dipping 3) Over-dipping of reagent strip - will result in leaching of reagents out of pads; briefly, but completely dip the reagent strip into the urine the kidneys are the major regulators of the acid-base content in the body. - A healthy individual will usually produce first morning specimen with a slightly acidic pH of 5.0 to 6.0, the pH of normal random samples can range from 5.0 to 8.0. principle of test is based on the double-indicator system of methyl red and CLINICAL SIGNIFICANCE OF URINE pH o A highly acidic urine pH occurs in: Acidosis Uncontrolled diabetes Diarrhea Starvation and dehydration Respiratory diseases in which carbon dioxide retention occurs and acidosis develops o A highly alkaline urine occurs in: Urinary tract obstruction Pyloric obstruction Salicylate intoxication Renal tubular acidosis Chronic renal failure Respiratory diseases that involve hyperventilation (blowing off carbon dioxide and the development of alkalosis) Source of error: A number of preanalytical variables can affect urine pH. Bacterial overgrowth in a specimen standing at room temperature will often lead to a higher pH due to the conversion of urea to ammonia. Diets with a high content of vegetables and citrus fruits may produce an alkaline pH. As a routine chemical tests performed on urine the most indicative of renal disease is the protein determination. The presence of proteinuria is often associated with early renal disease. Normal urine contains very little protein; usually, less than 10 mg/dl or 150 mg per 24 hours is excreted. Benign proteinuria is usually transient and can be produced by conditions such as: exposure to cold, strenuous exercise, high fever, dehydration, and in the acute phase of sever illnesses. Negative Trace + (30 mg/dL) ++ (100 mg/dL) +++ (300 mg/dL) ++++ (2000 mg/dL) Test :HEAT & ACETIC ACID TEST. Principle:proteins are denatured & coagulated on heating to give white cloud precipitate. Method:take 2/3 of test tube with urine, heat only the upper part keeping lower part as control. Presence of phosphates, carbonates, proteins gives a white cloud formation. Add acetic acid 1-2 drops, if the cloud persists it indicates it is protein(acetic acid dissolves the carbonates/phosphates) Bence Jones protein is a monoclonal globulin protein. Finding this protein in blood and urine is often suggestive of multiple myeloma or Waldenstrom's macroglobulinemia. Test:Proteins has unusual property of precipitating at (400 -600c) & then dissolving when the urine is brought to boiling(1000c) & reappears when the urine is cooled. clinical sign:The Bence-Jones protein urine test is used mainly to diagnose and monitor multiple myeloma, a blood cancer Source of error of protein:1- increase of Exercise . 2- increase eating of red meat….why?? Glucose test is the most frequent chemical analysis performed on urine because of its value in the detection and monitoring of diabetes mellitus. principle of test is based on a double sequential enzyme reaction :-Two very different tests are utilized by laboratories to measure urinary glucose, the glucose oxidase procedure provides a specific test for glucose. Negative CLINICAL SIGNIFICANCE OF URINE GLUCOSE: • Diabetes mellitus. • Renal glycosuria. Trace (100 mg/dL) + (250 mg/dL) SOURCE OF Error : 1- take a lot of diet have sugar 2-Drug and some disease that cause increase sugar ++ (500 mg/dL) +++ (1000 mg/dL) ++++ (2000+ mg/dL) Test:- BENEDICT’S TEST(semi quantitative) Principle:benedict’s reagent contains cuso4.In the presence of reducing sugars cupric ions are converted to cuprous oxide which is hastened by heating, to give the color. Method:take 5ml of benedict’s reagent in a test tube, add 8drops of urine. Boil the mixture. The term ketones represents; three intermediate products of fat metabolism, namely, acetone, acetoacetic acid, and beta-hydroxybutyric acid. The three ketone bodies are not present in equal amounts in urine. principle of test is based on the reaction of acetoacetic acid with sodium nitroprusside in a strongly basic medium . Acetoacetic Acid + Nitroprusside ------> Colored Complex CLINICAL SIGNIFICANCE OF URINE KETONES: • • Diabetic ketoacidosis Prolonged fasting TEST:Principle:acetone & acetoacetic acid react with sodium nitroprusside in the presence of alkali to produce purple colour. Method:- take 5ml of urine in a test tube & saturate it with ammonium sulphate. Then add one crystal of sodium nitroprusside. Then gently add 0.5ml of liquor ammonia along the sides of the test tube. - Change in colour indicates + test Negative Trace (5 mg/dL) + (15 mg/dL) ++ (40 mg/dL) +++ (80 mg/dL) ++++ (160+ mg/dL) OCCULT BLOOD :The term “occult” means “hidden,” and the methods used to test for blood in the urine are capable of detecting even minute amounts not visualized macroscopically. Another reason for this title is that these procedures actually detect the free hemoglobin from lysed red blood cells (RBCs). principle of test is based on the pseudoperoxidase action of hemoglobin and erythrocytes which catalyzes the reaction of 3, 3’, 5, 5’-tetramethyl-benzidine and buffered organic peroxide . CLINICAL SIGNIFICANCE OF URINE BLOOD :• Hematuria • Hemoglobinuria • Myoglobinuria TEST:- BENZIDINE TEST Principle:Principle-The peroxidase activity of hemoglobin decomposes hydrogen peroxide releasing nascent oxygen which in turn oxidizes benzidine to give blue color. Method:- mix 2ml of benzidine solution with 2ml of hydrogen peroxide in a test tube. Take 2ml of urine & add 2ml of above mixture. A blue color indicates + reaction. BILIRUBIN AND UROBILINOGEN:Bilirubin is formed from the breakdown of hemoglobin in the reticuloendothelial system. It is then bound to albumin and transported through the blood to the liver. In the intestines, bacterial enzymes convert bilirubin, through a group of intermediate compounds, to several related compounds which are collectively referred to as Urobilinogen . SCREENING TESTS FOR BILIRUBIN (BILE) Bilirubin can be detected in the urine before other clinical symptoms are present or recognizable Negative + (weak) ++ (moderate) +++ (strong) CLINICAL SIGNIFICANCE OF URINE bilirubin :• Increased direct bilirubin (correlates with urobilinogen and serum bilirubin). TEST:Ictotest (more sensitive tablet version of same assay) Serum test for total and direct bilirubin is more informative SCREENING TESTS FOR UROBILINOGEN:Screening for urobilinogen is useful in the diagnosis of liver function disorders. 0.2 mg/dL 1 mg/dL 2 mg/dL 4 mg/dL 8 mg/dL FOAM TEST:the urine is a yellowish-brown or greenish-yellow color and bilirubin is suspected, shake the urine. If a yellow or greenish-yellow foam develops, then bilirubin is most likely present. Bilirubin alters the surface tension of urine and foam will develop after shaking. REAGENT TEST STRIPS:Screening tests for urobilinogen are based on the Ehrlich Aldehyde Reaction: p-Dimethylaminobenzaldehyde + urobiligen = red-colored azo dye o CLINICAL SIGNIFICANCE OF URINE BLOOD :• High: increased hepatic processing of bilirubin . • Low: bile obstruction . White blood cells can be present in any body fluid depending on a cause for their presence. The most common white blood cell seen in a urine sample is the neutrophil, which is normally present in low numbers. Increased numbers of neutrophils usually indicate the presence of a urinary tract infection; and their presence is indicated by a positive leukocyte esterase test. Screening for urinary tract infections also includes evaluation of pH, protein, and nitrite A produces a lavender to with a reporting range of values from trace to large. Values reflecting cell numbers from negative to 500 may be reported. These results may not correlate with the numbers of neutrophils seen during microscopic examination. False-Positive Results: Strong oxidizing agents cause a false-positive leukocyte esterase result The nitrite test: is a rapid, indirect method for the early detection of significant and symptomatic bacteriuria.Common organisms that can cause urinary tract infections, such as Escherichia coli,Enterobacter, Citrobacter, Klebsiella,and Proteus species, produce enzymes that reduce urinary nitrate to nitrite. For this to occur, the urine must haveincubated in the bladder for a minimum of 4 hours. Hence,the first morning urine is the specimen of choice. Nitrite results are read at 30 or 60 seconds, depending on the manufacturer Pink spots or pink edges should not be considered a positive result. If the uniform pink color is very light, it may best be seen by placing the strip against a white background. The test is reported as positive or negative. Source of error of nitrite in urine: False-Positive Results The urine should be tested shortly after being voided, because if the urine is allowed to stand at room temperature for several hours, organisms may grow in the specimen and generate nitrite.9,10,48 Results may be misinterpreted as positive in urines that appear red or contain phenazopyridine and other substances that turn red in acid.5,11 False-Negative Results The sensitivity of the test is reduced in urine with a high specific gravity or elevated level of ascorbic acid.9,11 A negative test should never be interpreted as indicating the absence of bacterial infection. Interpretation: Nitrite results are read at 30 or 60 seconds, depending on the manufacturer. Any degree of uniform pink color should be interpreted as a positive nitrite test suggesting the presence of 105 or more organisms per milliliter. The color development is not proportional to the number of bacteria present. Pink spots or pink edges should not be considered a positive result.2,5,9,11 If the uniform pink color is very light, it may best be seen by placing the strip against a white background. The test is reported as positive or negative CLINICAL SIGNIFICANCE for nitrite: Early detection of bacteria is important in order to prevent cystitis from developing into inflammation or infection involving the kidney and renal pelvis. The nitrite portion of the test strip can be used to screen individuals who are at risk for developing urinary tract infections, such as diabetics, persons with recurrent infections, or pregnant women. The test is also useful in evaluating the success of antibiotic therapy that is used to treat a bladder infection. Thanks for watching our presentation سعد داحشالعمري سالم داحشالعمري مشار يسلمان الخيبري مشعل محمدالشريف * Textbook: Graff’s Textbook of Routine Urinalysis and Body Fluids * 1. Multistix© 10 SG reagent strips [Color chart]. Tarrytown, NY: Bayer * Corporation Diagnostics Division, 1996. * 2. Multistix© 10 SG reagent strips [Package insert]. Tarrytown, NY: * Siemens Healthcare Diagnostics Inc, 2008. * 3. de Wardener HE. The Kidney. 3rd Ed. Boston: Little, Brown & Co, 1967. * 4. James GP, Bee DE, Fuller JB. Accuracy and precision of urinary pH * determinations using two commercially available dipsticks. Am J. Clin * Pathol 1978;70:368–374.