247987_RG1_5ChemBonding
advertisement
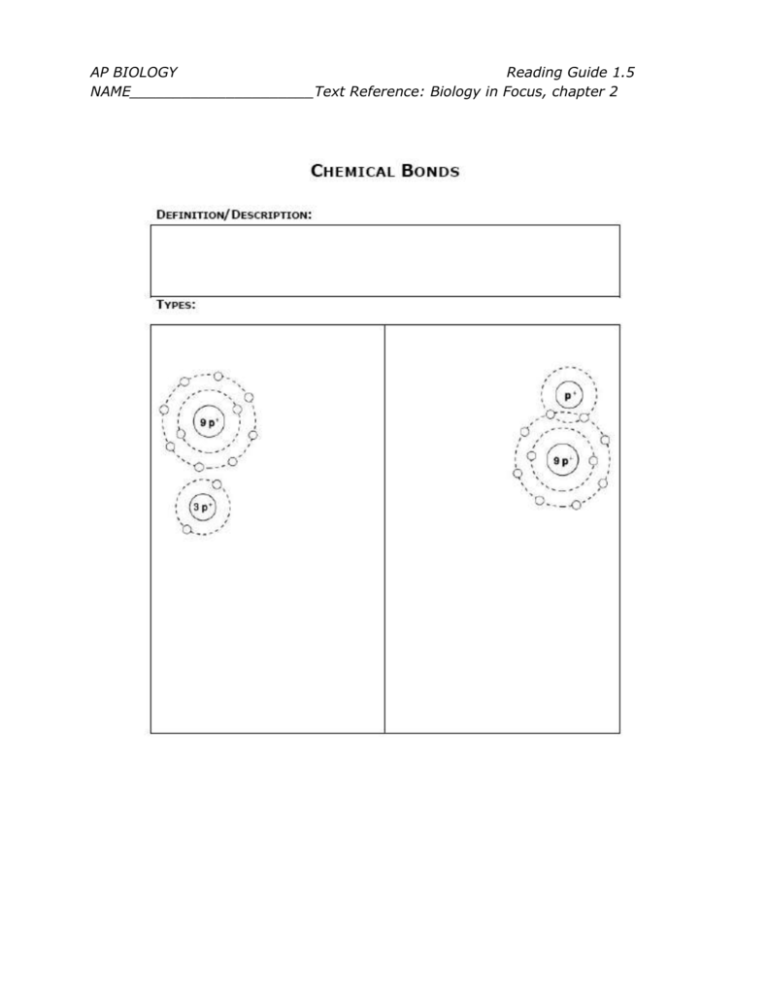
AP BIOLOGY Reading Guide 1.5 NAME_____________________Text Reference: Biology in Focus, chapter 2 AP BIOLOGY Reading Guide 1.5 NAME_____________________Text Reference: Biology in Focus, chapter 2 AP BIOLOGY Reading Guide 1.5 NAME_____________________Text Reference: Biology in Focus, chapter 2 QUESTIONS: 1. Define the following terms: Valence Electron Valence Shell 2. How is the chemical behavior of an atom determined by its electron configuration? 3. Why do atoms form chemical bonds? 4. Potential energy is the energy available to do work. Why do the electrons of an atom have potential energy? 5. Identify the type of bond described in each of the following. Use the key below to indicate your answers. A. Covalent, polar C. Hydrogen B. Covalent, nonpolar D. Ionic ______ Strongest bonds ______ Weakest type of bond ______ Bonds formed by the complete transfer of electrons from atom to another AP BIOLOGY Reading Guide 1.5 NAME_____________________Text Reference: Biology in Focus, chapter 2 ______ Bonds formed by the equal sharing of electrons between two atoms ______ Bonds formed by the unequal sharing of electrons between two atoms ______ Attraction between oppositely charged portions of two different polar molecules ______ C—C ______ Na—Cl ______ H—C ______ P—O ______ C—O ______ N—H 6. How can you determine if the bond between two atoms is polar covalent, nonpolar covalent, or ionic? 7. The distinction between inorganic and organic compounds is not always clear when dealing with open and closed systems, because everything is ultimately connected to everything else on the planet. Which of the following features of organic compounds is NOT found in inorganic compounds? A) oxygen B) hydrogen bonds C) ionizing chemical groups D) carbon atoms covalently bonded to each other 8. Match the description with the correct term. ______ Charged atom or molecule A. Anion ______ Negatively charged ion B. Cation ______ Positively charged ion C. Ion AP BIOLOGY Reading Guide 1.5 NAME_____________________Text Reference: Biology in Focus, chapter 2 9. How many electrons are shared between the atoms in each of the following: 10. What is the biological importance of weak bonds? 11. What is the meaning of the following statement: “Nonpolar covalent bonds and ionic bonds are two extremes of a continuum.”