Moraj
advertisement

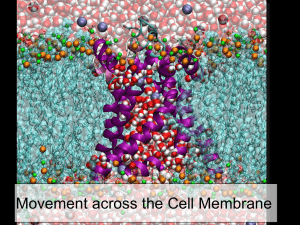
Manoj S. Nair, Ph.D Postodoctoral Fellow, Biochemistry 812 Biosciences bldg, 484 w. 12th ave Columbus, OH 43210 Nair.30@osu.edu 1. 2. 3. Types of transport across membranes Passive transport Carrier mediated Active transport Nernst equilibrium for ion transport Mechanism of ion transport (K-selectivity filter) Endocytosis of proteins/protein domains Introduction to Cellular Biophysics A. Molecular Basis of Membrane Transport. Essential Cell Biology Alberts, Bray, et al. Transport up a concentration gradient Uses energy (ATP) May also cause charge gradient across the membrane causing the molecule to move against the membrane potential. Properties of “Active” membrane pumps Example of a Na+/K+ pump ATPases (sometimes GTPases) Na+/K+ pump uses 30% resting ATP Active Pumps are used to transport materials against their electrochemical gradient Essential Cell Biology Alberts, Bray, et al a) Uniport: 1 type of solute is transported Eg: Valinomycin (K+ transport) V alinom ycin H 3C N CH3 CH O CH C O O H C CH H3C L -valine H C O N H C H CH CH 3 H3C D -hydroxy- isovaleric acid C CH3 O O CH 3 CH3 D -valine C L -lactic acid Valinomycin is a carrier for K+. It is a circular molecule, made up of 3 repeats of the sequence shown above. Puckering of the ring, stabilized by H-bonds, allows valinomycin V a lin o m y cin to closely surround a single O + unhydrated K ion. O O + Six oxygen atoms of the K ionophore interact with the O O + bound K , replacing O atoms of O waters of hydration. H yd rop h ob ic Valinomycin is highly selective for K+ relative to Na+. The smaller Na+ ion cannot simultaneously interact with all 6 oxygen atoms within valinomycin. Thus it is energetically less favorable for Na+ to shed its waters of hydration to form a complex with valinomycin. V a lin o m y cin O O K O O + O O H yd rop h ob ic Whereas the interior of the valinomycin-K+ complex is polar, the surface of the complex is hydrophobic. This allows valinomycin to enter the lipid core of the bilayer, to solubilize K+ within this hydrophobic milieu. Crystal structure V al-K K + V al-K + + K V al + V al m em brane Valinomycin is a passive carrier for K+. It can bind or release K+ when it encounters the membrane surface. Valinomycin can catalyze net K+ transport because it can translocate either in the complexed or uncomplexed state. The direction of net flux depends on the electrochemical K+ gradient. b) Symport: 2 different solutes transported together in one direction Eg: Glucose –Na+ tranporter in epithelial cells Lactose permease: H+ -lactose symport c)Antiport: 2 different solutes transport in opposite directions Eg: Adenine nucleotide translocase (ATP/ADP exchanger) Properties of transmembrane helices: Amphiphilic nature Designer Peptides of Ser & Leu: Formed a hexamer channel in phospholipid membranes. S.R.Goodman. 1998 What is the mechanism for ion selectivity of channels? This is a frontier of biophysics. With Passive Channels, ions or other substances move DOWN their electrochemical gradient + + - - - + + + - Basic structure of the potassium channel. Doyle et al. Science, 1998. Nobel Prize in Chemistry in 2003 KcsA Selectivity Filter • “Gated” channels i.e. channels that open in response to physiological stimuli Receptor-activated gate Essential Cell Biology Alberts, Bray, et al.