rcm7027-sup-0001-SI
advertisement

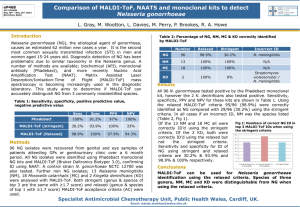
SUPPORTING INFORMATION GUMBOS matrices of variable hydrophobicity for matrix-assisted laser desorption/ionization mass spectrometry Hashim Al Ghafly, Noureen Siraj, Susmita Das, Bishnu P. Regmi, Paul K. S. Magut, Waduge Indika S. Galpothdeniya, Kermit K. Murray* and Isiah M. Warner* Figure S1. Powder X-ray diffraction pattern of [AP][Cl]. 1 Figure S2. Powder X-ray diffraction pattern of [AP][NTF2]. Figure S3. Powder X-ray diffraction pattern of [AP][Asc]. 2 Additional Experiments Microscopy. Microscopy was used to characterize the size of AP and AP-based GUMBOS on a MALDI target. Therefore, we have compared the sample morphology of our different compounds. The size of the matrix crystals are shown in the images depicted in Figure S4.These images were taken by using a Zeiss steREO Lumar.V12 stereomicroscope at a magnification of 150×. All samples were prepared on the MALDI target by using the dried-droplet method as described above. AP and AP-based GUMBOS formed smaller crystals. These crystals had sizes between 5 and 20 µm. Thus, one of the reasons for the enhanced performance of AP and APbased GUMBOS is likely the result of formation of smaller crystals. Results Figure S4. Images of different MALDI matrices in the presence of angiotensin II. The last image shows the empty target. The scale bar in each image represents 50 µm. 3 Figure S5. 1H NMR spectrum of AP (upper) and [AP][Cl] (lower). Figure S6. 1H NMR spectrum of (top) AP (green), (middle) ascorbic acid (red), and (bottom) [AP][Asc] (blue). 4 Figure S7. 1H NMR spectrum of [AP][NTf2]. Figure S8. 19F NMR spectrum of [AP][NTf2]. 5 Figure S9. Positive ion mode MALDI-TOF mass spectra for angiotensin II with CHCA. A B Figure S10. Positive ion mode MALDI-TOF mass spectra of (A) only AP and (B) angiotensin II with AP. A B Figure S11. Positive ion mode MALDI-TOF mass spectra of (A) only [AP][Cl] and (B) angiotensin II with [AP][Cl]. 6 A B Figure S12. Positive ion mode MALDI-TOF mass spectra of (A) only [AP][NTf2] and (B) angiotensin II with [AP][NTf2]. A B Figure S13. Positive ion mode MALDI-TOF mass spectra of (A) only [AP][Asc] and (B) angiotensin II with [AP][Asc]. Figure S14. Positive ion mode MALDI-TOF mass spectrum of CHCA with bradykinin. 7 Figure S15. Positive ion mode MALDI-TOF mass spectrum of bradykinin with [AP][Asc]. Figure S17. Positive ion mode MALDI-TOF mass spectrum of bradykinin with [AP]. Figure S16. Positive ion mode MALDI-TOF mass spectrum of bradykinin with [AP][Cl]. Figure S18. Positive ion mode MALDI-TOF mass spectrum of bradykinin with [AP][NTf2]. 8 Figure S19. Positive ion mode MALDI-TOF mass spectrum of CHCA with valinomycin. FigureS20. Positive ion mode MALDI-TOF mass spectrum of [AP][Asc] with valinomycin. Figure S22. Positive ion mode MALDI-TOF mass spectrum of AP with valinomycin. Figure S21. Positive ion mode MALDI-TOF mass spectrum of [AP][Cl] with valinomycin. Figure S13. Positive ion mode MALDI-TOF mass spectrum of [AP][NTf2] with valinomycin. 9 Figure S24. Positive ion mode MALDI-TOF mass spectrum CHCA with gramicidin. Figure S25. Positive ion mode MALDI-TOF mass spectrum of [AP][Asc] with gramicidin. Figure S26. Positive ion mode MALDI-TOF mass spectrum of [AP][Cl] with gramicidin. Figure S27. Positive ion mode MALDI-TOF mass spectrum of [AP] with gramicidin. Figure S28. Positive ion mode MALDI-TOF mass spectrum of [AP][NTf2] with gramicidin. 10 Figure S29. CHCA hydrophilic matrix detected higher intensity hydrophilic peptide (angiotensin II) compared with hydrophilic peptide (valinomycin). 11 Average ratio ( hydropholic /hydrophobic) Figure S30. [AP][Asc] hydrophilic matrix detected higher intensity hydrophilic peptide (angiotensin II) compared with hydrophilic peptide (valinomycin). Ratio (angiotension II/Valinomycin) 5 4 3 2 1 0 0.75 0.95 1.15 1.35 1.55 Log (partition coefficient) Figure S31. Ratio of average intensity (angiotensin II/valinomycin) versus log(Ko/w) of matrices. 12