ppt - Neurodynamics Lab
advertisement

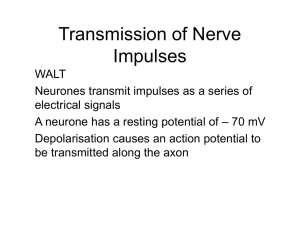
BME 6938 Mathematical Principles in Neuroscience Instructor: Dr Sachin S. Talahthi Excitable cells in the brain: Neurons Anatomy of a typical neuron More Detailed View of neuron anatomy The neuronal cell membrane Synapses Excitability of neuron Crash course on neuronal signaling Neurons communicate through electrical signaling Intracellular signaling is mediated through flow of ions through ion channels on cell membrane (Will discuss in details soon) Long distance cell to cell signaling is mediated through generation of action potentials that propagate along the axons (focus of neuronal modeling will be to understand the dynamical mechanism’s underlying the generation of action potential) Communication between neurons happen at synapses by the process of neurotransmission Conduction of nerve impulse Unmyelinated axon Animation of impulse propagation Myelinated axon Excitable properties of neuronal cell membrane: Intracellular signaling Essential fundamental laws in Cellular Neurophysiology: Ficks Law of Diffusion Ohms Law of Drift Space Charge Neutrality Fick’s Law of Diffusion Fick’s law relates the diffusion gradient of ions to their concentration. Ion Flux = Jdiff ¶ [C ] = -D ¶x Jdiff:Diffusion flux, measuring the amount of substance flowing across unit area per unit time ( molecules ) cm s D: Diffusion coefficient ( cms ) [C]: Concentration of the substance (ions) (molecules ) cm 2 Ficks Law Animation 2 3 Ohms law of drift (Microscopic view) Charged particle in the presence of external electrical field E experience a force resulting in their drift along the E field gradient Ion Drift = Jdrift ¶V = -z[C]m ¶x Jdrift:Drift flux, measuring the amount of substance flowing across unit area per unit time (molecules) cm s : electrical mobility of charged particle( ) [C]: Concentration of the substance (ions) ( molecules ) cm z:Valence of ion 2 3 Space charge neutrality Biological systems are overall electrically neutral; i.e., the total charge of cations in a given volume of biological material equals the total charge of anions in the same volume biological material å z [C ] = å z [C ] C i i A j i j j Some high-school chemistry 1 mole= Avogadro’s number (NA) of basic units (atoms, molecules, ions…) Concentration 1 is typically given in units of molar. Molar=1 mole/litre=10-3 moles/cm3 Relation between gas constant (R) and Boltzmann’s constant (k): R=kNA Faraday constant F: Magnitude of one mole of charged particles: F=qNA Some-algebra Membrane capacitance of a cell membrane is around 1 microF/cm2. Concentration of ions within and outside of a cell is 0.5 M. Determine the fraction of free (uncompensated) ions required to charge a spherical cell of radius 25 micro m to produce 100mV? Ans: ~ 0.000235% For realistic cell dimension, from above calculations we see that generation of 10s of mV of voltage does not violate space-charge neutrality (~99.9% of charges are compensated) Fundamental Equations of Cellular Neurophysiology Nernst-Plank Equation Goldman-Hodgkin-Katz Equation Are derived from the fundamental laws of neurophysiology that we talked about in lecture 2 ppt. Nernst-Plank Equation: Reversal Potential NPE describes the passive behavior of ion flow through biological cell membrane under the influence of concentration gradient and electric field Reversal Potential: (Nernst Equilibrium potential) RT æ [Cout ]ö Vm = V ( I = 0) = lnç ÷ zF è [Cin ] ø I=Current A/cm2; u=molar mobility cm2/V-sec-mol; F=Faraday constant (96480 C/mol) R=Gas constant (1.98 cal/oK-mol); C=Concentration molecules/cm3 Typical scale of reversal potential values Question: What is the direction of flow of following ions under normal conditions? 1.Na+ 2. K+ 3. Ca2+ 4. Cl(Hint: Look at the chart of reversal potentials and Nernst Equilibrium potential equation) At 37 oC mV Specific Examples-Nernst Potential and the need for active mechanism Ion concentration for cat motoneuron:Vm=-70 mV Inside mol/m3 Outside mol/m3 Na+ 15 150 K+ 150 5.5 Cl- 9 125 Nernst Potential: At body temperature 37oC VNa + [ ] [ ] æ Na + ö out = 62log10 ç ÷÷ = 62mV + ç Na è in ø VCl - VK + [ ] [ ] [ ] [ ] æ K+ ö out = 62log10 ç ÷÷ = -89 mV + ç K è in ø æ Cl - ö out = -62log10 ç ÷÷ = -70 mV ç Cl è in ø Gradient maintenance Active Transport: Flow of ions against concentration gradient. Requires some form of energy source Examples: Na+ pump Passive Transport: Selective permeability to some ions results in concentration gradient No energy source required Passive distribution of ions can be determined using the Donnan rule of equilibrium Graphical illustration of ionic current flow Donnan Equilibrium Rule The membrane potential equals the reversal potential of all ions that can passively permeate through the cell membrane. Mathematically the Donnan Rule implies: 1 m +m é COut ù é Cin-n ù ê C +m ú = ê C -n ú ë in û ë Out û 1 n Have a look at Donnan Rule in works; through animaltion developed by Larry Keeley: http://entochem.tamu.edu/Gibbs-Donnan/index.html Example: Application of Donnan Rule Consider a two compartment system separated by a membrane that is permeable to K+ and Cl- but is not permeable to a large anion A-. The initial concentrations on either side of membrane are: Ion type I (conc in mM) II (conc in mM) A- 100 0 K+ 150 150 Cl- 50 150 Is the system in electrochemical equilibrium (no ion flow across the membrane? If not, what direction the ions flow? And what are the final equilibrium concentrations? Goldman-Hodgkin-Katz Model Relates the current carried by ion’s across the cell membrane to the transmembrane potential. Can be derived as a solution to NPE equation under certain constraints: The cell membrane is homogeneous medium (uniform thin glass) Electric field across the cell membrane is constant Ion’s flow independently without interaction The flow of ion is affected by both concentration gradient and voltage difference across the membrane Goldman-Hodgkin-Katz Current Eqn Nonlinear I-V relationship for ionic current flow across a cell membrane under the influence of concentration and potential gradient uRT P= Fl V= zVF RT (P=permeability of ion) Goldman-Hodgkin-Katz Voltage Eqn Commonly known as the Goldman equation; is used to determine the membrane resting potential of a cell that is permeable to several ionic species. For membrane that is permeable to N positive ionic species and M negative ionic species: æ M + P + Ci å RT ç i C i E R = Vm (I = 0) = lnç F ç + P C + å i ç Ci è [ ] [ ] ö + å PC - C ÷ out j j ÷ N ÷ + P - Cj å Cj in out ÷ ø j N [ ] j in [ ] Application of the GHK equation Lets use GHK eqn to determine the contribution to membrane potential from active ion transport mechanism’s. Na-pump result in flow of 3 Na+ ions across the cell membrane for every 2K+ ions. What is the resulting equilibrium potential of the cell of squid axon for which the concentration gradients across the cell are: Ion type Inside (mM) Outside (mM) K+ 400 20 Na+ 50 550 The permeability ratio is Pk:Pna=1:.03