Electrochemical Measurement of Toxic Metal
advertisement

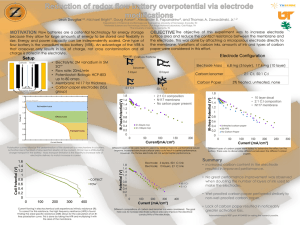
Electrochemical Measurement of Toxic Metal Contaminants in the Waters of the Golden Triangle Area By: Progga Chirontoni Mentor: Dr. Andrew Gomes Dan F. Smith Department of Chemical Engineering, Lamar University Texas STEM Conference-2014, Beaumont, TX Overview • About Heavy Metals • Detection Techniques • Nano-band electrode system and electrochemistry • Optimization • Results • Conclusion Heavy metals in the environment • Toxic metals such as lead, cadmium, copper and arsenic are referred to as heavy metals • Widespread Occurrence (regulated by federal and regional agencies) • Presence of chemical, petrochemical and metal-work industries in Golden Triangle area Effects on Human Health Metals Main source Health effects Maximum Permissible Limit (mg/L of water) Lead (Pb) natural deposits, plumbing of old households poor physical growth and 0.015 learning disabilities in children, kidney problems, and high blood pressure in adults Cadmium (Cd) phosphate fertilizers, iron, and steel industry, batteries Carcinogenic, kidney problems, poor growth rate, anemia and hypertension 0.005 Copper (Cu) household plumbing materials and industrial manufacture Gastro-intestinal distress and in the long run, experience liver or kidney damage. 1.30 Arsenic (As) volcanoes, weathering of arsenic-containing minerals and ores skin and internal cancers, diabetes and cardiovascular diseases 0.010 Detection Techniques LABORATORY-BASED 1. Spectrometric techniques (Hydride generation atomic absorption spectrometry (HG-AAS), Graphite furnace atomic absorption spectrometry (GFAAS), Atomic fluorescence spectrometry (AFS)) 2. Inductively coupled plasma (ICP) techniques (ICP-Atomic emission spectrometry (AES), ICP-Mass Spectrometry (MS)) 3. High performance liquid chromatography (HPLC) and ICP-MS 4. Laser induced breakdown spectroscopy (LIBS) FIELD-DEPLOYABLE 1. X-ray fluorescence 2. Colorimetric assays (spectrophotometers) 3. Electrochemical methods (Polargraphic techniques, Cathodic stripping voltammetry (CSV), Anodic stripping voltammetry (ASV)) Anodic Stripping Voltammetry (ASV) • Principle Deposition step: 𝑃𝑏 2+ + 2𝑒 − → 𝑃𝑏 0 𝐶𝑑 2+ + 2𝑒 − → 𝐶𝑑 0 𝐶𝑢2+ + 2𝑒 − → 𝐶𝑢0 𝐴𝑠 3+ + 3𝑒 − → 𝐴𝑠 0 Stripping step: 𝑃𝑏 0 𝐶𝑑 0 𝐶𝑢0 𝐴𝑠 0 → 𝑃𝑏 2+ + 2𝑒 − → 𝐶𝑑2+ + 2𝑒 − → 𝐶𝑢2+ + 2𝑒 − → 𝐴𝑠 3+ + 3𝑒 − ADVANTAGES OF ASV Large linear concentration range- from few mg/L to 0.1μg/L. Sensitivity of less than 0.1 ppb Selectivity Matrix effect immunity to samples with high ionic content Automated analysis and battery powered portable devices can be developed Extremely safe for monitoring, does not require vigorous heating, concentrated acid, etc. 7. Rapid analysis (10-15 min) 8. Inexpensive Analysis 1. 2. 3. 4. 5. 6. DISADVANTAGES OF ASV 1. As(V) in the sample has to be chemically reduced to As(III), increasing the sample analysis time. 2. Interferences Instrumentation- Nano-band electrode system • Nano-Band™ Explorer Portable instrument • Explorer Software to operate the instrument • Iridium electrode (for Lead, cadmium, copper) Carbon Nano-Band™ Electrode (for Arsenic) • Auxiliary electrode (Platinum) • Reference electrode (Ag/AgCl) Pictures of the Nano-Band electrode developed and fabricated at TraceDetect http://www.envirotechpubs.com/pdf/iet/2005/03/iet200503_046.pdf Advantages of Nanoelectrodes • Enhanced mass transport • Signal amplification • Greater number of measurement points • Great scope for parallel measurements • No requirement of removal of dissolved oxygen • More inert and much less sensitive to accidental overvoltage conditions Disadvantages of Nanoelectrodes • Surface-fouling • Fragility Procedure • Cleaning the Electrodes • Electrode Set up and Thin film plating - Carbon electrode and gold plating solution for As - Iridium electrode and mercury plating solution for Pb, Cd and Cu • Conditioning • Verification • Screening the sample for dissolved metal ion • Method of Standard addition Method of Standard Addition Voltammograms for lead standards (left) and the calibration curve (right). Optimization: Deposition Potential and Plate Time Stripping Current Vs Plate time 180 160 140 120 100 80 60 40 20 0 400 Stripping Current (nA) Stripping current (nA) Effect of deposition potential on stripping current 350 300 250 200 150 100 50 0 0 -200 -400 -600 -800 -1000 Deposition potential (mV) Arsenic (III) stripping current vs. deposition potential 0 30 60 90 120 150 180 210 Plate Time (sec) Arsenic (III) stripping current vs. plate time Optimization: Effect of Supporting Electrolyte Concentration Hydrochloric acid conc. vs Stripping current Stripping current (nA) 80 70 60 50 40 30 20 10 0 0 0.5 1 1.5 2 2.5 Hydrochloric acid conc. (Molarity) Plot of Stripping current of As (III) versus the HCL concentration Interference Peaks • The concentration of copper metal in drinking water is higher than other metals • So ASV scans usually have Copper interference peaks Ways to remove interference: • KI solution • Peak separation and Analysis software ASV scan of 20 ppb arsenic (III) in 2 M HCl having copper interference peak around 450 mV Sampling • • • • • Samples were collected from 1. 29 different locations in the Golden Triangle area. From both upstream and downstream Neches river and Sabine lake, samples were collected. pH and conductivity were measured. Hydrochloric acid was added until their pH was 2. Filtered with PTFE membrane filter. 2. 1. Neches river 2. Filtration of sample Sample Locations Sampling locations [A-E] (about every 5 miles upstream) Results Metals Sample A (ppb) Sample B (ppb) Sample C (ppb) Sample D (ppb) Sample E (ppb) Beaumont Port Tap water Arthur Tap (ppb) water (ppb) Max. Permissible limit (ppb) Pb 3.4 2.8 4.2 8.1 4.9 2.9 4.2 15.0 Cd ** ** 0.004 0.075 ** ** ** 5.000 Cu 379.4 168.0 573.5 686.2 298.3 312.7 483.6 1300.0 Concentration of heavy metals found in water samples in parts per billion ** Below detection limit Arsenic could not be detected in any of the samples Conclusions • Heavy metals such as Pb, Cd, Cu are present in the waters of golden triangle area • Within the permissible limit determined by EPA • No immediate danger of metal contamination in this area • Should be monitored in both day and night Future Works • • • Extend this research outside Golden Triangle Area in South Texas Analysis of organic chemicals in waters of Golden triangle area: organoarsenic, atrazine, diazinon, metalachor, and trenbolone Explore different detection techniques like Liquid chromatography and Mass spectrometric methods Acknowledgements • Department of Chemical Engineering, Lamar University • Department of Chemistry and Biochemistry, Lamar University • Office for Undergraduate Research (OUR), Lamar University • Research Enhancement Grant, College of Engineering References • Bryan, G.W., W.J. Langston, “Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries; a review”, • Environmental Pollution, 76 (1992), pp. 89–131. • Millward, G.E., A. Turner, Metal pollution ,in: J.H. Steele, S.A. Thorpe, S.A. Turekian Encyclopedia of Ocean SciencesAcademic Press, San Diego, CA (2001), pp. 1730–1737. • CSEM (Case Studies in Environmental Medicine), Agency for Toxic Substances and Disease Registry, Lead Toxicity, WB 1105, August 20 (2010). • Hem, J.D. Water Resources Res (1972), 8, 661-679. • Environmental Protection Agency, www.epa.gov. • Texas Annual Water quality report 2012, City of Beaumont, Water Utilities Department.