Respiratory system
advertisement

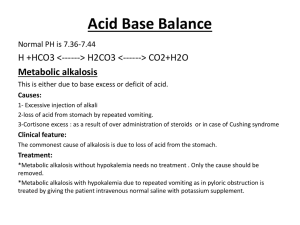
Ameer Aghbar Fayzeh Mohamad Group 1 Hitham Mosmar Wasef Sayeh pH , Acidity , and Alkalinity The degree of acidity or alkalinity of a solution is determined by its hydrogen ion [H ⁺]. Increase [H ⁺] acidic , decrease [H ⁺] basic . [H ⁺] in the body fluids is very small = 0.0000001 mol/L = 10 mol/L . Acidity and Alkalinity is expressed as the negative logarithm of the hydrogen ion [H ⁺] , which is referred to as pH pH , Acidity , and Alkalinity cont; To express hydrogen ion concentration as a pH , we use the role : pH= -log [H ⁺] e.g. : if [H ⁺] is 10 ⁻ ⁷ mol/L . To calculate the pH we dropped the negative sign and the pH is indicated as 7 : pH=-log[10 ⁻ ⁷ ]= 7 pH , Acidity , and Alkalinity cont; There is an inverse relationship between pH and the [H ⁺].As the [H ⁺] increases , the pH decreases , and vice versa . Acidic solution → low pH & high [H ⁺] . Alkaline solution → high pH & low [H ⁺]. Importance of Acid-Base Balance Normal metabolic function can occur only if the composition of the body cells and their surrounding environment are kept relatively constant . Necessary metabolic activities can only proceed if the balance between acidic and basic substances in body fluids kept within proper limis . The activities of virtually all enzymes within the cells are to some extent pH-dependant . Importance of Acid-Base Balance Cont ; In acidic or basic environment , some reactions are accelerated while others are slowed don or stopped completely . Hydrogen Ion Production And Excretion Acid-Base balance refer to homeostasis or [H⁺] in the body fluids . Products of the body’s metabolic reactions are acidic →the body produce large excess of acid under physiological conditions . If acids accumulate → the pH of blood and other component would be dramatic and lethal . Continual production of acids →need mechanisms to minimize the pH changes → these mechanisms must be able to respond to pH variation and accordingly . Mechanisms of pH Regulation and Hydrogen Ion Excretion The mechanisms are : respiratory system , renal system , and the chemical buffer system. Buffer system Buffer: chemical solution prevent excessive change in pH and hydrogen ion concentration when acid or base is added to the solution. Specifically buffer is mixture of a weak acid and alkali salt or weak base and acidic salt. Buffer can be take up any excess H+ that can produced in same way that sponge soaks up water. Buffering is only a short term solution To the problem of excess H+ , the body must get rid of the H+ by renal excretion or by lung without pH of blood dropped. Four main buffer systems in the body : • Bicarbonate/carbonic acid buffer system . • Phosphate buffer system • Protein buffer system • Hemoglobin buffer system . Bicarbonate \carbonic acid buffer system: -it is the most important and major one . -it buffers 70% of the fixed acid in plasma and 30% of fixed acid in RBCs . -it can be regulated by both kidney and lung. -Carbonic acid (H₂CO₃) can be retained or exhaled as CO₂ (by the lung). -bicarbonate (HCO₃) can be retained or excreted by the kidney tubules as required. The normal ratio in blood between HCO₃\H₂CO₃= 20\1 , so the system is easly buffered against excess acid production. Two kinds of acids : • Volatile acids: that can be excreted as gases by the lung. • Non volatile acids : that can be excreted by the kidney , for example : lactic acid , keton bodies, phosphoric acid , sulfuric acid. Respiratory system: CO₂ is the major end product of metabolism, formed continuously in the cell . From the cell diffuse in to interstitial fluid to the blood stream where it form H₂CO₃ and dissolve it . RBCs transport CO₂ to the lung as H₂CO₃ (which it is in the blood stream now. RBCs transport CO₂ to the lung as H₂CO₃ (which it is in the blood stream now. The Respiratory System In the lung : CO₂ is reformed and diffuse in to alveoli , to be exhaled. So the result : CO₂ exhaled by the lung , H₂O will dissipated into the general water pool of the body. NOTE: the equilibrium reaction can be shifted to the left : to retain more H ⁺ , and can be shifted to the right : to eliminate acid as CO₂ as required by the body. If H+ concentration is imbalanced : the respiratory center will stimulated; • if [H+] is high (acidic pH) increase in the rate of respiration .hyperventilation ( that mean: eliminate more acid as CO₂) • if [H+] is low (alkaline pH) decrease rate of respiration hypoventilation ( that mean retain more acid as CO₂) in the lung equilibrium reaction can be shifted to the left or to the right . Respiratory center can respond to the changes in hydrogen ion concentration and blood pH very rapid . The lung can adjust the [H ⁺] with second after a sudden changes. The lung alone can’t return the pH to its normal level of 7.4 so the kidney must act to restore the balance. Renal system: Any imbalance in [H ⁺] kidney tubules cells are stimulated to adjust the excretion or reabsorption (retention) of acid as H ⁺ ions , or buffer as HCO3 ions . If pH is acidic (low) kidney respond by excreting more H ⁺ and retain more HCO₃ to neutralize excess acid . pH is alkaline kidney will retain H ⁺ ion and excrete HCO₃ . Renal response to [H ⁺] changes and blood pH is slow in comparison to the respiratory system. Renal system work alone so need more time (days or more ) to adjust [H ⁺] . Kidney is more powerful of the control mechanisms and 100% efficiency rate. By themselves : kidney are able to retain pH to completely normal given adequate time . Acid – Base Disturbances Acidosis and Alkalosis Acidosis and alkalosis occur when an imbalance in the normal blood pH of the body occurs. An increase in blood pH level results to alkalosis and a decrease results in acidosis. The organs which work to restore Homeostasis : • Kidney >>> H ⁺ and HCO₃⁻ • Lungs >>> CO₂ The importance of H₂CO₃ and HCO₃⁻ . PH is directly proportional to the ratio HCO₃⁻ / H₂CO₃ . Acidosis / Alkalosis CO ₂ + H₂O H₂CO₃ H ⁺ + HCO₃⁻ Acidosis Acidosis : is a condition in which there is excessive acid in the body fluids . The PH < 7.35 / increase in [ H+ ] General causes : • Certain diseases like emphysema or kidney failures • If you starve yourself or if you have uncontrolled diabetes • If your protein intake is in excess • Ingestion of a large amount of acidic substances Alkalosis Alkalosis : is a condition in which the body fluids have excess base (alkali). General causes : • Severe diarrhea causes excessive loss of body fluid. • Loss of carbonic acid due to rapid breathing ( hyperventilation ). • Excessive vomiting leads to loss of hydrochloric acid. • Excessive intake of sodium bicarbonate, to relieve the acid in stomach. Irritability Muscle weakness Symptoms Muscle spasms Muscle twitches Respiratory / Metabolic Metabolic >>>>>> kidney >>>>>> HCO₃⁻ Respiratory >>>>> lungs >>>> H2CO3 Arterial Blood Gas (ABG) is a blood test that is performed using blood from an artery . involves puncturing an artery with a thin needle and syringe and drawing a small volume of blood. The radial artery. is used to determine • PH • PCO₂ • PO₂ • HCO₃⁻ level • Concentration of lactate , HB , electrolytes , oxyHB , carboxyHB and methemoglobin In human anatomy, the radial artery is the main blood vessel , with oxygenated blood , of the lateral aspect of the forearm. Arterial Blood Gas analyzing machine. Respiratory or Metabolic The most two effective systems in regulating pH: • Respiratory system: volatile substances(e.g:CO2). • Renal system :non-volatile substances(e.g. : lactate ,ketone bodies ,phosphoric acid and sulfuric acid). Metabolic Disturbances Problem either in the excretion or retaining of H ⁺and HCO₃⁻ in the kidneys It might be either acidosis or alkalosis 1-Acidosis: This occurs due to excess of non-volatile acids or loss base. In the normal situations kidneys must get rid of H ⁺ in this case in order to reduce the concentration of acids. In the abnormal way : the kidneys can’t excrete as much H ⁺ as needed -----Metabolic Acidosis How to recognize Metabolic Acidosis The metabolic component in determining the acidity or alkalinity is HCO₃⁻ . If [ HCO₃⁻] decreases------drop in pH---- metabolic acidosis pH=pKa+ log(HCO₃⁻/H₂CO₃) If HCO₃⁻/H₂CO₃ is less than 20 so we will have metabolic acidosis. Metabolic alkalosis Decrease of non-volatile acids or increase in the concentration of bases . The normal situation: the kidneys will retain H+ and excrete HCO₃⁻ How could we recognize this: [HCO₃⁻] increases------pH increases-----Metabolic alkalosis Summary of metabolic disturbances *If [HCO₃⁻] decreases---drop pH---Metabolic Acidosis. *If [HCO₃⁻] increases---increase in pH--Metabolic Alkalosis. Respiratory Determined by the partial pressure of CO₂ These acid -base disturbances occurs due to changes in the [CO₂] which will change the [H₂CO₃]. CO₂+H₂O H₂CO₃ It might be either acidosis o alkalosis 1- Acidosis: Increase in the partial pressure of CO₂-------increase in [H₂CO₃]----increase Acidity ----- decrease pH Respiratory alkalosis It occurs due to decrease in the partial pressure of CO2 ---- decrease in [H2CO3]--increase alkalinity----increase in pH Respiratory acid – base disturbances *If PCO₂ increases---increase in [H₂CO₃]----acidosis. *If PCO₂ decreases--decrease in [H₂CO₃]-- alkalosis. Normal values of pH,[HCO₃] and Pressure of CO₂ Item Normal range Unit pH 7.35-7.4-7.45 ------------- 36-40-44 mmHg 90-100 mmHg Actual bicarbonate HCO3 22-24-26 Mmol/1 Standard HCO₃ 22-24-26 Mmol/1 PCO₂ P O₂ Mixed Disturbances Mixed disturbances include both respiratory and metabolic ( acidosis and alkalosis) . In mixed disturbances we usually have a primary disturbance that causes the major effect on pH and a compensatory disturbance that tries to retain the pH to its normal value(runs in the opposite direction). E.g.: if we have pH=7.10 and we have respiratory acidosis(increase PCO₂) and metabolic alkalosis ( increase in [HCO₃]). The major one disturbance: respiratory . Example on a mixed disturbance caused by a drug An overdose of Salicylate ( aspirine)------leads to hyperventilation( excessive elimination of CO₂)-----respiratory alkalosis. The metabolism of salicylate---accumulation of acids----metabolic acidosis Some diseases lead to acid- base disturbances In the normal situation the systems of the body can keep up with changes in the acidity or alkalinity of the blood by its buffering systems and this will keep pH 7.35-7.45 . Acid-base disturbances that will cause a shift in blood pH outside its normal range is called uncompensated and usually result in the cases of some common diseases(due to accumulation of large amounts of acids or bases) . Some diseases lead to acidosis Metabolic acidosis Respiratory Acidosis Uncontrolled DM(increase in ketoacids) Lung disease-COPD-retention of CO₂ Starvation or sever reduced carbohydrates Head injury leads to depression in the diet (increase in ketoacids) respiratory center----retention of CO₂ Sever exercise ( increase in lactic acid) Sever diarrhea (GI loss of HCO₃) Renal failure (failure in the excretion of H ⁺ ) Salicylate overdose Shock (increase in lactate) Any condition results in hypoventilation Common conditions lead to alkalosis Metabolic alkalosis Excessive administration of sodium bicarbonate during treatment of cardiac arrest (increase in HCO₃⁻) Respirator alkalosis High fever (stimulation of respiratory center)------loss of CO₂ Prolonged vomiting (excessive gastric loss Hysteria (hyperventilation ---loss of CO₂) of H ⁺) Hyperaldosteronism (renal loss of H ⁺) Any condition leading to hyperventilation Ameer Aghbar Fayzeh Mohamad Group 1 Hitham Mosmar Wasef Sayeh