synthesis and characterization of polyacrylonitrile
advertisement

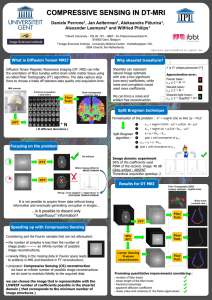
SYNTHESIS AND CHARACTERIZATION OF POLYACRYLONITRILE (PAN) AND CARBON FIBERS Prof. Dr. Tahir Jamil Chairman Engr. Shahzad Maqsood Khan Presenter Department of Polymer Engineering & Technology University of the Punjab, Lahore PRESENTATION APPROACH ? ? ? Carbon Fiber PAN Polymer Polymer POLYMER THE SCIENCE AND ENGINEERING OF LARGE MOLECULES • Long chain molecules • long molecule made up by the repetition of small unit called monomers BUILDIGNG BLOCK POLYMER AT PLAY Find Polymers in figure POLYMER CLASSES Polymers Inorganic Natural Clays, Sands, Glass, Rocklike, Ceramics, Graphite/Diamond, Silicas Synthetic Fibrous glass, Silicon Carbide, Poly(boron nitrid), Poly(sulfur nitride) Organic Organic/Inorganic Siloxane, Polyphosphazenes, Polyphosphate esters, Polysilanes, Sol-gel network Natural Proteins, Nucleic acids, Lignins, Polysccharides, Polyisoprene, Melanin Synthetic PE, PS, Nylons, PET, PVC, PU, PC, PMMA, PVAC, PP, PTFE POLYMER -A-A-A-A-A-A-A-A- Homo Polymer -A-B-B-A-B-A-A-B- Random Copolymer -A-B-A-B-A-B-A-B- Alternating Copolymer -A-A-A-A-B-B-B-B- Block Copolymer -A-A-A-A-A-A-A-AB-B-B-B-B-B- Graft Copolymer POLYMER POLYACRYLONITRILE (PAN) IMPORTANCE OF PAN Homo polymers of Polyacrylonitrile have been used as • Fibers in hot gas filtration systems • Outdoor awnings • Sails for yachts • Fiber reinforced concrete Mostly copolymers containing Polyacrylonitrile are used as • Fibers to make knitted clothing, like socks and sweaters • Outdoor products like tents POLYACRYLONITRILE (PAN) • In 1893 Acrylonitrile was prepared by reacting Propylene with Ammonia (NH3) and oxygen in the presence of catalysts. • PAN is a vinyl polymer and a derivative of the acrylate family of polymers. • It is made from acrylonitrile monomer through suspension methods using free-radical initiators. POLYACRYLONITRILE (PAN) PAN LAB SYNTHESIS • Polymerization of acrylonitrile (AN) by redox method • Flask or lab reactor • Nitrogen atmosphere • Fitted with a condenser • Reaction medium (Dimethylsulfoxide (DMSO) solvent or water) PAN LAB SYNTHESIS • Emulsifier ( e.g Sodium bisulfite (SBS) ) • Initiators ( e.g Potassium Persulfate (KPS), Azodiisobutyronitrile (AIBN), Itaconic acid (IA) ) • Time 1–3.5 hr • Precipitation • Filtration • Washing ( methanol and deionized water etc) • Drying under vacuum till a constant weight PAN CHARACTERIZATION • FTIR (Fourier Transform Infrared Spectrophotometer) • NMR (Neutron Magnetic Resonance) • GPC (Gel Permeation Chromatograph) • DSC (Differential Scanning Calorimeter) • TGA (Thermo Gravimetric Analyzer) • TMA (Thermo Mechanical Analyzer) PAN CHARACTERIZATION FTIR R. Setnescu, S. Jipa, T. Setnescu, W. Kappel, S. Kobayashi, Z. Osawa. IR and X-ray characterization of the ferromagnetic phase of pyrolysed polyacrylonitrile, Carbon 37, (1999) 1–6. PAN CHARACTERIZATION NMR 1H-NMR spectrum of the PAN precursors 13C-NMR spectrum of the PAN precursors PAN CHARACTERIZATION DSC N. Yusof and A. F. Ismail. Preparation and characterization of polyacrylonitrile/acrylamide-based activated carbon fibers developed using a solvent-free coagulation process, International Journal of Chemical and Environmental Engineering. 1, (2010) 79-84. PAN CHARACTERIZATION TGA H. B. Sadeghi, H. A. Panahi, M. Abdouss, B. Esmaiilpour, M. N. Nezhati, E. Moniri, Z. Azizi. Modification and Characterization of Polyacrylonitrile Fiber by Chelating Ligand for Preconcentration and Determination of Neodymium Ion in Biological and Environmental Samples. J. APPL. POLYM. SCI. (2013) 1125-1130. PAN CHARACTERIZATION TMA T. V. Sreekumar, T. Liu, B. G. Min, H. Guo, S. Kumar, R. H. Hauge, R. E. Smalley, Polyacrylonitrile Single Walled Carbon Nanotube Composite Fibres. Adv. Mater. 16, (2004) 58-61. PAN CHARACTERIZATION DMA Temperature v/s Storage Modulus of PAN PAN CHARACTERIZATION DMA Temperature v/s Tanδ of PAN PAN INDUSTRIAL PRODUCTION POLYMER SYNTHESIS PILOT PLANT DEPARTMENT OF POLYMER ENGG. PU LHR POLYMER SYNTHESIS PILOT PLANT DEPARTMENT OF POLYMER ENGG. PU LHR POLYMER SYNTHESIS PILOT PLANT DEPARTMENT OF POLYMER ENGG. PU LHR POLYMER SYNTHESIS PILOT PLANT DEPARTMENT OF POLYMER ENGG. PU LHR PAN FIBER & CARBON FIBER IMPORTANCE OF PAN FIBER PAN-based fibers eventually supplanted most rayon-based fibers, and they still dominate the world market. In addition to high modulus fibers, researchers have also developed a low modulus fiber from PAN that had extremely high tensile strength. Used in • Sporting goods such as golf clubs, tennis rackets, fishing rods, and skis • Military • Commercial aircrafts IMPORTANCE OF CARBON FIBER Strength: carbon fibers tensile strength is un-matched by any metal available (Titanium alloys, Cr Mo, steel or Aluminum alloys) Weight: carbon fiber/epoxy weight per volume is less than half that of aluminum almost 4 times lighter than titanium Fatigue resistance of carbon fiber surpasses that of any other structural material IMPORTANCE OF CARBON FIBER Yield strength: carbon fiber has a very high yield strength allowing it to flex under extreme loading and return to its original shape Corrosion: carbon fiber/epoxy is extremely resistant to corrosion IMPORTANCE OF CARBON FIBER Carbon fiber parts will be lighter and stronger. Because of such properties you find this technology used in • Aviation • Sports • High-end racing and • Snowmobiles CARBON FIBER Carbon fibers are derived from one of the three precursor materials • PAN (Polyacrylonitrile fiber) • PITCH • Isotropic • Mesophase • Rayon PAN FIBER INDUSTRIAL PRODUCTION • Melt Spinning • Dry Spinning • Wet Spinning • Wet/Dry Spinning PAN FIBER FORMATION Polyacrylonitrile fibers were produced by wet-spinning. The coagulation bath is normally • DMSO/H2O system, • Bath temperature is 60°C • Bath concentration is 65% (namely, DMSO/H2O=65/35(wt/wt)) • Bath minus stretch ratio is –10% PAN FIBER INDUSTRIAL PRODUCTION PAN FIBER INDUSTRIAL PRODUCTION PAN FIBER INDUSTRIAL PRODUCTION PAN FIBER INDUSTRIAL PRODUCTION CARBON FIBER FORMATION Fiber changing color. The white PAN strands at the bottom pass through the air heated oven and begin to darken. Quite quickly they turn to black CARBON FIBER INDUSTRIAL PRODUCTION PAN FIBER INDUSTRIAL FORMATION • Oxidization • Stress graphitization of Polyacrylonitrile based carbon fiber • Carbonization (graphitization) PAN FIBER INDUSTRIAL FORMATION Oxidization • This produces an oxidized ladder polymer structure approximately parallel to the fiber axis which may be regarded as the template for the formation of the oriented carbon fiber. • Oxidation involves heating the fibers to around 300 oC in air. This evolves hydrogen from the fibers and adds less volatile oxygen. • The polymer changes from a ladder to a stable ring structure, and the fiber changes color from white though brown to black. CARBON FIBER INDUSTRIAL PRODUCTION Stress Graphitization of Polyacrylonitrile Based Carbon Fiber • Carbon fiber can be made by the pyrolysis of organic polymer fiber precursors. The strength of PAN carbon fiber declines when heated above 1,200° C. • Therefore increasing strength with Young's modulus can be obtained if stress is applied to the fiber at graphitizing temperatures. CARBON FIBER INDUSTRIAL PRODUCTION CARBONIZATION (GRAPHITIZATION) • Involves heating the fibers up to 3000 oC in an inert atmosphere. • Fibers are now nearly 100 % carbon CARBON FIBER FORMATION CHEMISTRY When we heat Polyacrylonitrile, the heat causes the cyano repeat units to form cycles… CARBON FIBER FORMATION CHEMISTRY At higher temperature, carbon atoms kick off their hydrogen, and the rings become aromatic. This polymer is a series of fused pyridine rings. This expels hydrogen gas, and gives us a ribbon-like fused ring polymer. CARBON FIBER FORMATION CHEMISTRY When the temperature increases from 600 up to 1300 oC, the ribbons will themselves join together to form even wider ribbons like this: CARBON FIBER FORMATION CHEMISTRY More nitrogen is expelled and the ribbons are really wide, and most of the nitrogen is gone, leaving us with ribbons that are almost pure carbon in graphite form. the FIBER CHARACTERIZATION • XRD (X Ray Diffraction) • SEM (Scanning Electron Microscopy) • DSC (Differential Scanning Calorimeter) • TGA (Thermo Gravimetric Analyzer) • DMA (Dynamic Mechanical Analyzer) • UTM (Universal Testing Machine) PAN TO CARBON FIBER CHARACTERIZATION XRD PAN TO CARBON FIBER CHARACTERIZATION XRD DATA PAN TO CARBON FIBER CHARACTERIZATION FTIR PAN CHARACTERIZATION YOUNG’S MODULUS & TENSILE STRENGTH GASES RELEASED DURING PYROLYSIS OF PAN ELEMENTAL ANALYSIS PAN FIBER GRADE The Carbonization temperature will determine the grade of fiber produced: Carbonization Temperature (oC) to 1000 1000 - 1500 1500 - 2000 2000 + (Graphitizatio n) Grade of Carbon Fiber Low Modulus Standard Modulus Intermediate Modulus High Modulus Modulus of Elasticity (GPa) to 200 200 - 250 250 - 325 325 + CARBON FIBER GROUPING FINAL HEAT TREATMENT TEMPERATURE Type-I, high-heat-treatment carbon fibers (HTT) Final heat treatment temperature > 2000C and can be associated with high-modulus type fiber. Type-II, intermediate-heat-treatment carbon fibers (IHT) Final heat treatment temperature should be > = 1500C and can be associated with high-strength type fiber. Type-III, low-heat-treatment carbon fibers Final heat treatment temperatures not greater than 1000C. These are low modulus and low strength materials. CARBON FIBER FORM PAN FIBER CARBON FIBER FROM PITCH MECHANICAL PROPERTIES OF CARBON FIBER OUR RESEARCH PAPER 61 Journal of Pakistan Institute of Chemical Engineers Vol. XXXVII Synthesis And Characterization of Polyacrylonitrile Copolymers Waqar Ahmad, Shahzad Maqood Khan, Muhammad Arif Butt and Tahir Jamil* Abstract Polyacrylonitrile (PAN) and copolymers of PAN with monomers like MMA, BA, VA, AM, AA, and S of varying compositions and molecular weights were prepared by emulsion polymerization in a continuous aqueous phase in the presence of sodium lauryl sulfate as emulsifier and potassium persulfate/ammonium persulfate as initiator. The molecular weights were determined from the dilute solution viscosity using MarkHouwink equation. The chemical compositions of copolymers were characterized by FTIR spectroscopy. Keywords: Polyacrylonitrile (PAN), Polymer, Emulsion polymerization, FT-IR CONCLUSIONS OF OUR WORK • The synthesis of homo and copolymers of PAN via emulsion polymerization was successfully achieved • Maximum yield of 94.5 % for Polyacrylonitrile (100) • Maximum yield of 90.2 % for P(AN-co-AM-co- MAA, 96.1:3.2:0.7) • The highest molecular weight, Mv = 144068, for copolymer P(AN-co-AM-coMAA, 96.1:3.2:0.7) • followed by Mv = 75403.85 for P(AN-MMA, 96:4) • and Mv = 75403.69 for PAN (100). • MMA was found to be the best monomer for copolymerization of AN. CONCLUSIONS OF OUR WORK • As commercially available PAN precursor for carbon fiber have molecular weight about 150000 and we have achieved 144068 MW for P(AN-co- AM-co-MAA, 96.1:3.2:0.7), this sample of PAN can be a very suitable precursor for carbon fiber. ACKNOWLEGMENT We are grateful to • Prof. Dr. Arshad Chughtai (Chairman, Department of Textile Engineering & Technology University of the Punjab Lahore • Miss. Nafisa Gull (Research Officer, Department of Polymer Engineering & Technology University of the Punjab Lahore) • Dr. Misbah Sultan (Assistant Professor, Department of Polymer Engineering & Technology University of the Punjab Lahore) • Engr. Muhammad Shafiq, Engr. Aneela Sabir (Lecturer, Department of Polymer Engineering & Technology University of the Punjab Lahore) • Miss Saba Bahzad Khan (Lab Supervisor, Department of Polymer Engineering & Technology University of the Punjab Lahore) • Engr, Adnan Ahmed, Engr. Muhammad Azeem Munawar, Engr. Khurram Javed, Miss Sidra Waheed (Research Technician, Department of Polymer Engineering & Technology University of the Punjab Lahore) THANKS FOR YOUR ATTENTION ! NO QUESTIONS PLZ BUT STILL YOU ARE WELCOME FOR QUESTIONING