Document
advertisement
Chem 261: Inorganic Chemistry
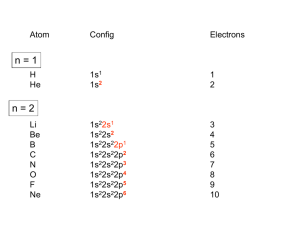
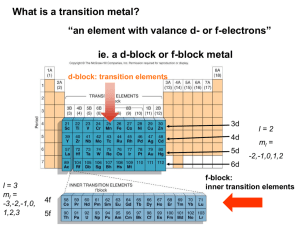
The elements in the periodic table are often divided into four categories:
(1) main group elements, (2) transition metals, (3) lanthanides, and
(4) actinides.
How do we determine the electronic configuration of the central metal ion in
any complex?
Try to recognise all the entities making up the complex and knowing
whether the ligands are neutral or anionic, so that you can determine the
oxidation state of the metal ion.
A simple procedure exists for the M(II) case.
22
23
24
25
26
27
28
29
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
4
5
6
7
8
9
Cross off the first 2,
2
3
Evaluating the oxidation state
Oxidation States and their Relative Stabilities:
Why do these elements exhibit a variety of oxidation states?
Because of the closeness of the 3d and 4s energy states.
The most prevalent oxidation numbers are shown in green.
Sc
+3
Ti
+1
+2
+3
+4
V
+1
+2
+3
+4
+5
Cr
+1
+2
+3
+4
+5
+6
Mn +1
+2
+3
+4
+5
+6
Fe
+1
+2
+3
+4
+5
+6
Co
+1
+2
+3
+4
+5
Ni
+1
+2
+3
+4
Cu
+1
+2
+3
Zn
+2
+7
An increase in the number of oxidation states from Sc to Mn.
All seven oxidation states are exhibited by Mn.
There is a decrease in the number of oxidation states from Mn to Zn.
WHY?
Because the pairing of d-electrons occurs after Mn (Hund's rule) which in
turn decreases the number of available unpaired electrons and hence, the
number of oxidation states.
The stability of higher oxidation states decreases in moving from Sc to Zn.
Mn(VII) and Fe(VI) are powerful oxidizing agents and the higher oxidation
states of Co, Ni and Zn are unknown.
The relative stability of +2 state with respect to higher oxidation states,
particularly +3 state increases in moving from left to right.
This is justifiable since it will be increasingly difficult to remove the third
electron from the d-orbital.
Summary of Physical Properties
1. have large charge/radius ratio;
2. are hard and have high densities;
3. have high melting and boiling points;
4. form compounds which are often paramagnetic;
5. show variable oxidation states;
6. form coloured ions and compounds;
7. form compounds with profound catalytic activity
8. form stable complexes.
Coordination Chemistry
A coordination compound, sometimes called a coordination complex,
contains a central metal atom or ion surrounded by a number of oppositely
charged ions or neutral molecules (possessing lone pairs of electrons)
which are known as ligands.
If a ligand is capable of forming more than one bond with the central
metal atom or ion, then ring structures are produced which are known as
metal chelates, the ring forming groups are described as chelating agents
or polydentate ligands.
The coordination number of the central metal atom or ion is the total
number of sites occupied by ligands.
Note: a bidentate ligand uses two sites, a tridentate three sites etc.
Ligands:
molecular
formula
Lewis
base/ligand
Lewis
acid
donor
atom
coordination
number
[Zn(CN)4]2-
CN-
Zn2+
C
4
[PtCl6]2-
Cl-
Pt4+
Cl
6
[Ni(NH3)6]2+
NH3
Ni2+
N
6
Mono-dentate
Multidentate Ligands
Chelating ligands bonded to metal – rings – chelate rings - any number of
atoms in the ring.
most common – five or six atoms, including metal.
Coordination Numbers and Geometries
Isomers
Primarily in coordination numbers 4 and 6.
Arrangement of ligands in space, but also the ligands themselves.
Ionization isomers
Isomers can produce different ions in solution e.g.
[PtCl2(NH3)4]Br2
[PtBr2(NH3)4]Cl2
Polymerization isomers
Same stoichiometry, different arrangement in space.
Eight compounds with formula Co(NH3)3(NO2)3.
Coordination isomers
In compounds, both cation and anion are complex, the distribution of ligands
can vary, giving rise to isomers.
[Co(NH3)6]3+ [Cr(CN)6]-3
Linkage isomers
e.g. Nitro and nitito, N or O
coordination possible
and
[Cr(NH3)6]+3 [Co(CN)6]-3
Geometric isomers
Formula is the same but the
arrangement in 3-D space is
different e.g. square planar
molecules give cis and trans
isomers.
For hexacoordinate systems other species can also occur.
For M(X)3(Y)3 systems there is
facial and meridian
Are “stereo” isomers also possible? An analogy to organic chirality.
Molecules which can rotate light.
Enantiomers (non-superimposable mirror images)
Complex Stabilities
In aqueous solution a comparison of metal complexes and their affinity for
the H2O molecule as a competing ligand has been studied. Here are some
general observations:
•
For a given metal and ligand, complexes where the metal oxidation
state is +3 are more stable than +2.
• Stabilities of complexes of the first row of transition metals vary in
reverse of their cationic radii (in general)
MnII < FeII < CoII < NiII > CuII > ZnII
• Hard and soft Lewis acid-base theory
• Chelate Effect - effect is the additional stability of a complex containing a
chelating ligand, relative to that of a complex containing monodentate
ligands with the same type and number of donors as in the chelate.
Mainly an entropy effect.
Cu(H2O)4(NH3)2]2+ + en = [Cu(H2O)4(en)]2+ + 2 NH3
When ammonia molecule dissociates - swept off in solution and the
probability of returning is remote.
When one amine group of en dissociates from complex ligand retained by
end still attached so the nitrogen atom cannot move away – swings back
and attach to metal again.
Therefore the complex has a smaller probability of dissociating.
The origin of colour - absorption
The colour can change depending on a number of factors e.g.
• Metal charge
• Ligand
Physical phenomenon
Are there any simple theories to explain the colours in transition
metal complexes?
There is a simple electrostatic model used by chemists to rationalize
the observed results
Bonding in Transition Metal Complexes
Covalent bonds by sharing pairs of electrons was
first proposed by G. N. Lewis in 1902.
It was not until 1927, however, that Walter Heitler and
Fritz London showed how the sharing of pairs of electrons
holds a covalent molecule together.
The Heitler-London model of covalent bonds was the basis
of the valence-bond theory.
The last major step in the evolution of this theory was the
suggestion by Linus Pauling that atomic orbitals mix to form
hybrid orbitals, such as the sp, sp2, sp3, dsp3, and d2sp3 orbitals.
How do we view this and do we really need to ?
Valence-Bond Theory
It is easy to apply the valence-bond theory to some
coordination complexes, such as the Co3+ complexes below.
d2sp3- inner sphere complex low spin complex
sp3d2- outer sphere complex high spin complex
Note: Such a situation will not arise for d1, d2 and d3
ion configuration.
Deficiencies of VB approach to bonding
• Assumes that all d orbitals in a complex are equal in energy.
• The arbitrary use of 3d and 4d orbitals for bonding energy differential
ignored.
• The theory is unable to adequately explain electronic and magnetic
properties of complexes.
•VBT is widely used in organic and main group element chemistry.
•In TM metal chemistry VBT is superseded by the Crystal Field Theory
(CFT).
The Crystal-Field Theory
Crystal Field Theory is based on the idea that a purely electrostatic
interaction exists between the central metal ion and the ligands.
Covalent bonding is ignored.
Crystal field theory was developed by considering two compounds:
manganese(II) oxide, MnO octahedral geometry,
copper(I) chloride, CuCl tetrahedral geometry.
We will start with octahedral and then expand to tetrahedral and square
planar complexes
The five d-orbitals in an octahedral field of ligands
Tsuchida noticed a trend in while looking at a series
of Cobalt(III) Complexes.
With the general formula : [Co(NH3)5X]
He arrived a series which illustrates the effect of
ligands on Do (10Dq)
He called it:
The Spectrochemical Series
Tsuchida, R. Bull. Chem. Soc. Jpn. 1938, 13, 388
The magnitude of the splitting
(ligand effect)
Strong
field
Weak
field
The energy gap between t2g and eg levels is designated Do or
10Dq
The spectrochemical series
CO, CN- > phen > NO2- > en > NH3 > NCS- > H2O > F- > RCO2- > OH- > Cl- >
Br- > I-
The magnitude of the splitting
(metal ion effect)
Strong
field
Weak
field
D increases with increasing formal charge on the metal ion
D increases on going down the periodic table
Splitting of d orbitals in an octahedral field
eg
3/5 Do
Do
2/5 Do
t2g
CFSE(Oh) = (–0.4x + 0.6y )Do + nP
E(t2g) = -0.4Do x 3 = -1.2Do
E(eg) = +0.6Do x 2 = +1.2Do
Placing electrons in d orbitals
d5
1 u.e.
5 u.e.
d6
0 u.e.
4 u.e.
d8
2 u.e.
2 u.e.
d7
1 u.e.
3 u.e.
d9
1 u.e.
1 u.e.
d10
0 u.e.
0 u.e.
Crystal–Field Stabilisation Energy (CFSE)
Q. Determine which of the following are more likely to be high spin
complexes:
[Fe(CN)6]3[FeF6]3[Co(H2O)6]+3
[Co(CN)6]-3
[Co(NH3)6]+3
[Co(en)3]+3
Solution: Compare the ligands on the spectrochemical series. Since we want
a high spin complex, we want weak field ligands. The weaker field ligands in
the above are H2O and F-, so complexes 2 and 3 are more likely to be high
spin. (The cyanide complexes are least likely)
When the 4th electron is assigned it will either go into the higher
energy eg orbital at an energy cost of D0 or be paired at an
energy cost of P, the pairing energy.
d4
Strong field =
Low spin
(2 unpaired)
Weak field =
High spin
(4 unpaired)
P < Do
P > Do
Notes: the pairing energy, P, is made up of two parts. 1)
Coulombic repulsion energy caused by having two electrons in
same orbital
Pairing Energy, P
The pairing energy, P, is made up of two parts.
1) Coulombic repulsion energy caused by having two electrons in
same orbital. Destabilizing energy contribution of Pc for each
doubly occupied orbital.
2) Exchange stabilizing energy for each pair of electrons having
the same spin and same energy. Stabilizing contribution of Pe
for each pair having same spin and same energy
P = sum of all Pc and Pe interactions
Another way to view the energy in textbooks
The energy increase of the eg orbitals and the energy decrease of
the t2g orbitals must be balanced relative to the energy of the
hypothetical spherical field (sometimes called the barycenter)
The energy of the eg set rises by +3/5Do = +6Dq while the
energy of the t2g set falls by –2/5Do = –4Dq, resulting in no
net energy change for the system.
DE = E(eg)8 + E(t2g)9
= (2)(+3/5Do) + (3)(–2/5Do)
= (2)(+6Dq) + (3)(–4Dq) = 0
The magnitude of Do depends upon both the metal ion and the attaching
ligands
Magnitudes of Do are typically ~100 – 400 kJ/mol (~8,375 –
33,500 cm–1)
11 kJ/mol = 83.7 cm–1
Most aquo complexes are high spin, because H2O is a weak field
ligand.
Almost all Co3+ (d6) complexes are low spin, including
[Co(H2O)6]3+, except [CoF6]3–, which is high spin.
Second and third row transition metal ions tend to have low
spin states - These ions tend to have larger Do values
Larger 4d and 5d orbitals result in smaller P values, owing
to lesser electronic repulsions
4d and 5d orbitals overlap with ligand orbitals, delocalizing
electron density onto the ligands - Can we calculate or guestimate Do?
In comparing groups
of similar ligands
rationalize the order.
These effects have been
placed on a semiquantitative basis by
Jorgensen who assigned
a factor g to a sampling
of metal ions and a
factor f:
Δo ≈ g x f x 1000 cm-1
-
High- spin d 4
t2g3 eg1
x=3,y=1
E = (0.4x – 0.6y)Δo = 0.6 Δo
E = (0.4x – 0.6y)Δo = 1.6 Δo + P
Low- spin d 4
t2g4 eg0
x=4,y=0
Results and Observations
1. Doctahedral gets larger for increasing oxidation state
2. It increases down a group
e.g. Co < Rh < Ir
3. With a given ligand and oxidation state Doctahedral
varies irregularly across the first row transition
metals
The spectrochemical series
The splitting of d orbitals in the crystal field model not only
depends on the geometry of the complex, it also depends on
the nature of the metal ion, the charge on this ion, and the
ligands that surround the metal. When the geometry and
the ligands are held constant, this splitting decreases in the
following order.
For metals the series is:
Pt4+ > Ir3+ > Rh3+ > Co3+ > Cr3+ > Fe3+ > Fe2+ > Co2+ >
Ni2+ > Mn2+
When the geometry and the metal are held constant,
the splitting of the d-orbitals increases in the
following order
For ligands the series is:
I - < Br - < [NCS] - < Cl - < F - < - OH < NH3 < en < CN Weak field <
Increasing DO
< Strong field
Tetrahedral Coordination
Dt = 4/9Do
All tetrahedral compounds are
High Spin
The difference results in an energy split between the two levels by Dt
or10Dq'. Relative to the barycenter defined by the hypothetical spherical
field
" the e level is lower by –3Dt /5 = –6Dq'.
" the t2 level is higher by +2Dt /5 = +4Dq‘
In principle, both high and low spin configurations are conceivable for d 3–d 6
ML4 Td complexes
With extremely rare exceptions, only high spin configurations are observed.
" Dt is much smaller than Do
For a given ligand at the same M-L distances, it can be shown that Dt =
(4/9)Do
" Dt << P in ordinary complexes, so high spin is favoured
The crystal field stabilization energy for tetrahedral complexes is calculated
from the following equation:
CFSE(Td) = (–0.6x + 0.4y )Dt + nP
What is the LFSE for octahedral ions of the following configurations:
(a) d 3
(b) high-spin d 5
(a) electronic configuration : t2g3eg0, x = 3, y = 0
Therefore, LFSE = (0.4x – 0.6y)Δo = [(0.4)(3) – (0.6)(0)]Δo = 1.2 Δo
(b) electronic configuration : t2g3eg2, x = 3, y = 2
Therefore, LFSE = (0.4x – 0.6y)Δo = [(0.4)(3) – (0.6)(2)]Δo = 0
What is LFSE for both high- and low-spin d 6 configuration?
Δo is the difference in energy between eg and t2g.
The net energy of a t2gx egy configuration relative to the barycentre is
called the ligand field stabilization energy (LFSE).
LFSE = (0.4x – 0.6y)Δo
Let us see what happens when we withdraw the 2 trans ligands in an Oh
complex (let it be the z ligands)
When this happens, we have a tetragonally distorted octahedral
complex.
As soon as the distance from Mm+ to these 2 ligands becomes greater
than the other 4 ligands, new energy differences are established.
z2 orbital becomes more stable than x2-y2 orbital.
yz and xz are equivalent more stable than xy
dx2-y2
eg
Δo
E
dxy
dz2
t2g
dzy , dzx
Whether this happens depends on the metal ion and the ligands
concerned.
Square complexes of CoII, NiII and CuII lead to energy level diagrams
shown as follows:
M = CoII, NiII and CuII
dx2-y2
eg
Δo exactly
Δo
2/5 Δo
t2g
dz2
1/12 Δo
octahedral
MX6
square
MX4
dyz , dzx
The spectrochemical series
The splitting of d orbitals in the CF model not only depends on
the geometry of the complex, it also depends on the nature of the
metal ion, the charge on this ion and the ligands that surround
this ion.
When the geometry and the ligands are held constant, this
splitting decreases in the following order:
Pt4+ > Ir3+ > Rh3+ > Co3+ > Cr3+ > Fe3+ > Fe2+ > Co2+ > Ni2+ > Mn2+
When the geometry and the metal are held constant, the splitting
of the d- orbitals increases in the following order:
I- < Br- < [NCS]- < Cl-< F- < OH- < H2O < NH3 < en < CN- < CO
The ligand- field splitting parameter, Δo varies with the identity of the
ligand.
In the series of complexes [CoX(NH3)5]n+ with X = I-, Br-, Cl- H20 and
NH3, the colours range from purple (for X = I-) through pink (X = Cl-)
to yellow (with NH3).
This observation indicates that energy of the lowest electronic transition
increases as the ligands are varied along the series.
Ligand that give rise to high energy transition (such as CO) is referred
to as a strong-field ligand.
Ligands that give rise to low energy transitions (such as Br-) referred to
as weak-field ligand.
Tetragonally distorted complexes the Jahn Teller effect
The Jahn‐Teller (J‐T) theorem states that in molecules/ ions that have a
degenerate groundstate the molecule/ion will distort to remove the
degeneracy
Stretching of the two atoms in z‐direction in an octahedron leads to an
advantage for all orbitals with z‐components because the repulsion decreases
Splitting of the two energetic levels into four levels at all complexes with
unsymmetric occupation of the higher level d‐orbitals often show
Magnetic properties of metal complexes
Diamagnetic complexes
very small repulsive
interaction with external
magnetic field
no unpaired electrons
Paramagnetic complexes
attractive interaction with
external magnetic field
some unpaired electrons
s n(n 2)
The spin-only magnetic moment of a complex = µ
Defined as µ = 2 [ S(S+1)]1/2 µB
(µB = Bohr magneton = 9.274 x10-24 JT-1)
N.B. Each unpaired electron has a spin quantum = ½
Therefore for multi-electron systems S = ½ n, where n is the
number of unpaired electrons
From this we get µ = [n(n+2)]1/2 µB
Experimentally [Fe(OH2)6]3+ which is paramagnetic is
found to have a magnetic moment of 5.3 µ/µB
From the table this value corresponds to a value for 5
unpaired electrons i.e. a high-spin t2g3eg2 configuration
The numbers are never exact
The spin-only magnetic moment of a complex = µ
Defined as µ = 2 [ S(S+1)]1/2 µB
(µB = Bohr magneton = 9.274 x10-24 JT-1)
N.B. Each unpaired electron has a spin quantum = ½
Therefore for multi-electron systems S = ½ n, where n is the
number of unpaired electrons
From this we get µ = [n(n+2)]1/2 µB
Experimentally [Fe(OH2)6]3+ which is paramagnetic is
found to have a magnetic moment of 5.3 µ/µB
From the table this value corresponds to a value for 5
unpaired electrons i.e. a high-spin t2g3eg2 configuration
The numbers are never exact
Magnetic
measurements
Used to determine the number of unpaired spins in a complex, hence
identify its ground-state configuration.
Compounds are classified as diamagnetic if they are repelled by a
magnetic field and paramagnetic if they are accepted by a magnetic
field.
The spin-only magnetic moment, μ, of a complex with total spin
quantum number is given by:
μ = 2 {S (S + 1)}½ μB
μB = Bohr magneton
Measured magnetic moments include contributions from both spin and
orbital spin. In the first transition series complexes the orbital
contribution is small and usually ignored.
The magnetic moment of a certain Co(II) complex is 4.0 μB . What is
its d- electron configuration?
A Co(II) complex is d 7.
Two possible configurations: t2g5eg2 (high-spin, S = 1½) with 3
unpaired electrons or t2g6eg1 (Low-spin, S = ½) with 1 unpaired
electron.
The spin-only magnetic moments are 3.87 μB and 1.73 μB.
Therefore, the only consistent assignment is the high-spin
configuration t2g5eg2.
The magnetic moment of the complex [Mn(NCS)6]4- is 6.06 μB. What is
its electron configuration?